Aluminum (Al) is a/an:
o heterogeneous mixture
O compound
O homogeneous mixture
o element
Answers
Answer:
Element.
Explanation:
Aluminum is an element because it is not separable into simpler substances. One thing important to keep in mind is that if something is on the Periodic Table of Elements, it is 100% an element.
Related Questions
what is the correct name for Ga(NO2)3?
Answers
Gallium Nitrite your welcome
A binary III/V direct bandgap semiconductor called gallium nitride is ideally suited for high-power transistors that can function at high temperatures. It has been widely utilized in light-emitting diodes (LED) since the 1990s. Its chemical formula is Ga(NO₂)₃.
Blu-ray discs are read using a blue light that gallium nitride emits. Gallium nitride is also utilized in lasers, photonics, RF components, and semiconductor power devices. GaN will be used in sensor technology in the future.
Gallium nitride power amplifiers are perfect for microwave and terahertz (ThZ) devices, such as imaging and sensing, the aforementioned future market, due to their capacity to operate at significantly higher temperatures and voltages than gallium arsenide (GaAs) transistors.
To know more about Gallium nitride, visit;
https://brainly.com/question/29456292
#SPJ6
if answered correctly i will give brainlest

Answers
Answer: Earthquake location
Explanation:
Answer:
Volcano chains and arcs, and earthquake locations
Primary amines can be distinguished from secondary and tertiary amines by reacting with.
Answers
Primary amines can be distinguished from secondary and tertiary amines by reacting with nitrous acid (HNO2). The reaction with nitrous acid is known as the "Diazotization reaction" and is specific to primary amines.
This reaction allows for the conversion of primary amines into diazonium salts, which are highly unstable and can undergo further reactions to form various products.
In the diazotization reaction, primary amines react with nitrous acid to form a diazonium salt and water. The reaction proceeds as follows:
R-NH2 + HNO2 -> R-N2+X- + H2O
Secondary and tertiary amines do not undergo this reaction with nitrous acid because they lack the necessary hydrogen atom on the nitrogen atom to form the diazonium salt. Therefore, this reaction can be used as a distinguishing test to differentiate primary amines from secondary and tertiary amines.
The formation of the diazonium salt can be confirmed through various tests, such as the formation of colored precipitates or the coupling reactions with other compounds. These reactions are specific to primary amines and help in their identification and distinction from secondary and tertiary amines.
Learn more about Primary amines from the given link:
https://brainly.com/question/30887184
#SPJ11
Chemical disequilibrium is likely to be present in:_________
Answers
Chemical disequilibrium is likely to be present in any system where the forward and reverse reactions are not in balance.
This can occur in a variety of situations, such as when the reactants are not present in the correct proportions, when the reaction conditions are not ideal, or when there are external factors affecting the reaction. For example, in a chemical reaction where one product is constantly being removed from the system, the reaction may never reach equilibrium.
Similarly, in a reaction where the temperature or pressure is constantly changing, the equilibrium may shift in one direction, leading to a chemical disequilibrium. Ultimately, chemical disequilibrium occurs when a reaction is not able to maintain a stable equilibrium state. Chemical disequilibrium is likely to be present in environments where reactions are ongoing and not yet in a stable state. These situations can be found in systems experiencing changes in temperature, pressure, or concentrations of reactants and products. Examples include volcanic areas, hydrothermal vents, or chemical industries where continuous production or consumption of reactants occurs. The presence of chemical disequilibrium provides opportunities for further reactions to take place, leading to new products and potential energy releases. Understanding these environments can offer insights into various natural processes and technological applications.
To know about equilibrium:
https://brainly.com/question/30694482
#SPJ11
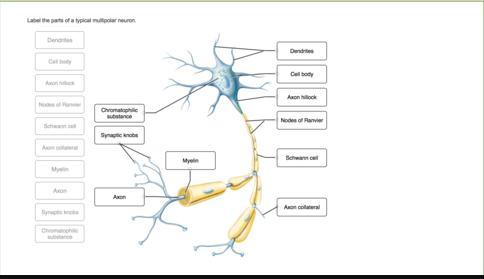
Choose the structure of the multipolar motor neuron that the function best fits by clicking and dragging the labels to the correct location
Answers
The structure of the multipolar motor neuron that the function best fits are found in the attachment.
What is a multipolar motor neuron?A multipolar motor neuron is a type of neuron that has many processes extending from the cell body.
Since it sends information from the central nervous system (CNS) to the body's muscles, organs, and glands, it is sometimes referred to as a multipolar efferent neuron.
The multipolar motor neurons are part of the motor system which are crucial for the regulation and coordination of both voluntary and involuntary movements.
Learn more about a multipolar motor neuron at: https://brainly.com/question/29762704
#SPJ1
is the source still usable? the half-life of 60co60co is 5.271 yearsyears . is the source still usable? the half-life of is 5.271 . yes no
Answers
No, the source is not usable. It is barely usable taking into account it's half life.
The period of time it takes for one-half of a radioactive isotope to decay is known as the half-life. An individual radioactive isotope's half-life is unaffected by environmental factors and is independent of the isotope's starting concentration. A substance's activity is a thermodynamic characteristic connected to its chemical potential. Chemical potentials are less practical to employ than activities, which are more closely tied to concentration measurements like partial pressures and mole fractions.
The activity is
\(\frac{dN}{dt} =\) λN
Therefore
N= \(N_{o} 2^{-\frac{t}{T_{0.5} } }\)
The amount of elapsed time since the source was created is roughly 2.5 years. Thus, we expect the current activity to be after calculating and using values,
= 3600 .
The source is barely usable.
Learn more about half Life
brainly.com/question/24710827
#SPJ4
Give the number of protons and the number of neutrons in the nucleus of the following isotopes: a) Carbon-14 b) Cobalt-60 c) Gold-197 d) Uranium-235
Answers
Explanation:
We are given different isotopes and we have to identify the number of protons and neutrons that are in the nuclueus of each atom.
a) Carbon-14:
By definition two isotopes are atoms that have the same atomic number but different mass number. The atomic number of an atom is equal to the number of protons of that atom, and the mass number is equal to the number of protons plus the number of neutrons.
atomic number = n° of protons
mass number = n° of protons + n° of neutrons
n° of protons = atomic number
n° of neutrons = mass number - n° of protons
n° of neutrons = mass number - atomic number
If two isotopes have the same atomic number but different mass number we can say that two isotopes have the same number of protons but different number of neutrons.
In we pay attention to carbon-14 we can look for its atomic number in the period table: 6. And its mass number is the one that we are given after the name of the element: 14.
n° of protons = atomic number = 6
n° of protons = 6
n° of neutrons = mass number - atomic number = 14 - 6
n° of neutrons = 8
b) Cobalt-60:
atomic number = 27 (from the periodic table)
mass number = 60
n° of protons = atomic number = 27
n° of protons = 27
n° of neutrons = mass number - atomic number = 60 - 27
n° of neutrons = 33
c) Gold-197:
atomic number = 79 (from the periodic table)
mass number = 197
n° of protons = atomic number = 79
n° of protons = 79
n° of neutrons = mass number - atomic number = 197 - 79
n° of neutrons = 118
d) Uranium-235:
atomic number = 92 (from the periodic table)
mass number = 235
n° of protons = atomic number = 92
n° of protons = 92
n° of neutrons = mass number - atomic number = 235 - 92
n° of neutrons = 143
Answer:
a) Carbon-14: n° of protons = 6 n° of neutrons = 8
b) Cobalt-60: n° of protons = 27 n° of neutrons = 33
c) Gold-197: n° of protons = 79 n° of neutrons = 118
d) Uranium-235: n° of protons = 92 n° of neutrons = 143
2. *
Which one of the following is the atomic number of an alkali metal?
A. 10 B. 11
C. 15
D. 20
A
B
Answers
Answer:
c
Explanation:
Name and draw skeletal formula of all the structural isomers of C4 H10 O that are alcohols.
Answers
One of the isomers of C4H10O that is an alcohol is the 1-butanol or butyl alcohol:
Another of the alcohols that has the given formula is tert-butanol or tert-butyl alcohol:
The third isomer of C4H10O is 2-butanol:
And the last isomer is isobutanol or isobutyl alcohol:




What is the molar ratio of hydrogen (H2) to iron (Fe)?

Answers
Answer: 3:2
Explanation:
Choose the paramagnetic species from below.
Ar
O
Ti4+
All of the above are paramagnetic.
None of the above are paramagnetic.
Answers
The correct answer is option (c) Ti4+.
The species which are attracted to a magnetic field are known as paramagnetic species. If we talk about the given options, then we can see that there are only 3 species that are given. Out of these three, only Ti4+ is paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic. If we talk about Ti4+, then it contains 2 unpaired electrons, which makes it paramagnetic. This is the reason why the correct answer is Ti4+.In Ar, all the electrons are paired, which makes it diamagnetic. In O, there are 2 unpaired electrons, which makes it paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic.
Learn more about paramagnetic species at brainly.com/question/29990302
#SPJ11
the fluid component of connective tissue is called ________.
Answers
The fluid component of connective tissue is called the extracellular matrix. The extracellular matrix (ECM) is a complex mixture of substances that fills the space between cells in connective tissue.
The extracellular matrix (ECM) is composed of a fluid component and various types of proteins, fibers, and other molecules. It is often referred to as tissue fluid or interstitial fluid. It is a clear, colorless fluid that fills the spaces within the connective tissue and provides a medium for the exchange of nutrients, gases, and waste products between cells and blood vessels.
The fluid in the ECM contains water, ions, small molecules, and dissolved substances such as hormones, enzymes, and nutrients. It also plays a role in maintaining the hydration and structural integrity of the tissue.
Learn more about extracellular matrix in:
https://brainly.com/question/14696607
#SPJ4
if the percent yield is 80.2%, what mass of k (in grams) is needed to obtain 27.6 g of h2? (assume in excess of hcl).
Answers
Stoichiometry and the idea of percent yield can be used to calculate the mass of K required to produce 27.6 g of H2. The reaction's balanced chemical equation is 2 K + 2 HCl 2 KCl + H2.
How much kclo3 is required to make 32.0 g of o2?Response and justification Hence, 126.23 g of potassium chlorate are needed to generate 32 g of oxygen.
How is yield determined in g?Divide the mass of the reactant by the molecular weight to get the mass per mole. As an alternative, we can multiply the liquid solution's density in grammes per millilitre by the amount of reactant solution in millilitres. Next, divide the outcome by the reactant's molar mass.
To know more about Stoichiometry visit:-
brainly.com/question/30215297
#SPJ1
Do particles vibrate more when they are hot or when they are cold?
Answers
Answer:
when they are cold
Explanation:
particles will only vibrate when they are hot due to heat transfer by conduction and temperature changes as well
MARK BRAINLIEST PLEASE....i will be glad ..thank you
Answer:
particles vibrate more when they are hot.
Explanation:
This is because the heat transfer from one particle to the other causes vibrations in particles of a substance..And if the substance is cold the particles will not move and will be held in a framework
i hope it will clear your concepts...
PLEASE HELP HELP ME. THIS IS DUE TODAY PLEASE

Answers
Answer:
B, C
Explanation:
Hope it helps i read it all
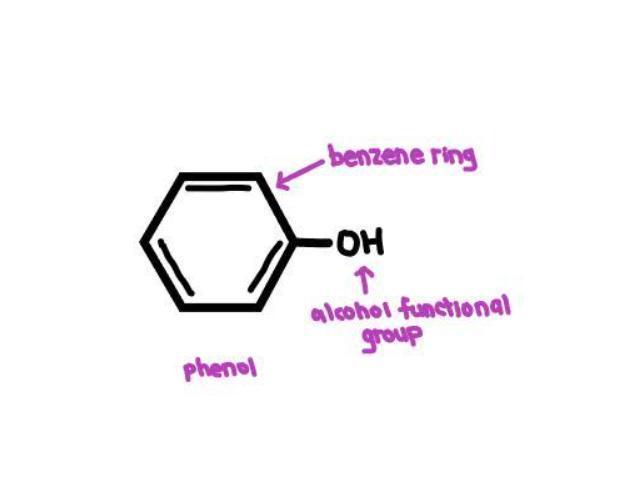
Name the compound when an alcohol is attached to an aromatic ring
Answers
Answer:
Phenol
Explanation:
A phenol is a compound that has an alcohol functional group (-OH) attached to a benzene ring.
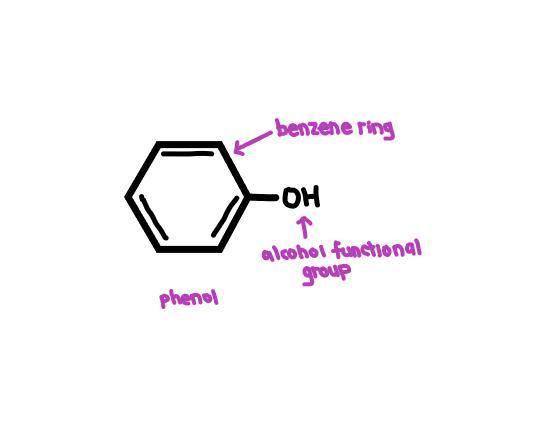
The compound formed when an alcohol is attached to an aromatic ring is called a phenol. Phenols are a type of organic compound that contain a hydroxyl group (-OH) attached to an aromatic hydrocarbon group. The hydroxyl group in phenols is directly attached to the carbon atom of the aromatic ring, and this makes phenols distinct from alcohols, which have a hydroxyl group attached to an alkyl group.
Phenols can be prepared by a variety of methods, including the reaction of an aryl halide with a metal hydroxide, such as sodium hydroxide (NaOH), or the reaction of an arene oxide with a proton source, such as an acid. One common method for preparing phenols is called the Dowd-Beckwith reaction, which involves the oxidation of an aromatic hydrocarbon using a peroxyacid, such as m-chloroperoxybenzoic acid (MCPBA).
Phenols have a wide range of applications in industry and in organic chemistry. They are used as disinfectants and antiseptics, as well as in the production of plastics, resins, and other chemicals. Phenols are also used in the synthesis of pharmaceuticals, dyes, and flavors.
To know more about phenol:
https://brainly.com/question/30898108
#SPJ11
Calculate the molar solubility and the solubility in g/L of each salt at 25 degreeC: (a) PbF2 Ksp = 4. 0 x 10^-8 ______ x 10^___ M ______ g/L (b) Ag2C03 Ksp = 8. 1 x 10^-12 ____ x 10^____ M ______ x 10^_____ g/L (c) Bi2S3 Ksp = 1. 6 x 10-72 ______ x 10^____ M _____ x 10^_____ g/L Enter all of your answers in scientific notation except the solubility of (a)
Answers
The Molar solubility and the solubility of each salt at 25°C.
(a) PbF₂ Ksp = 4.0 x 10⁻⁸ , 1.8 x 10⁻⁷ M, 4.41 x 10⁻⁵ g/L
(b) Ag₂CO₃ Ksp = 8.1 x 10⁻¹², 1.2 x 10⁻⁴ M, 0.0398 g/L
(c) Bi₂S₃ Ksp = 1.6 x 10⁻⁷² , 3.2 x 10⁻¹⁶M, 1.65 x 10⁻¹³ g/L
(a) PbF₂:
Ksp = [Pb₂+][F-]²
Let x be the molar solubility of PbF₂. Then, [Pb2+] = x and [F-] = 2x. Substituting into the Ksp expression and solving for x:
4.0 x 10⁻⁸ = x*(2x)²
x = 1.8 x 10⁻⁷ M
To convert to g/L, we need to multiply by the molar mass of PbF₂ (245.2 g/mol):
solubility = 1.8 x 10^-7 * 245.2 = 4.41 x 10⁻⁵ g/L
(b) Ag₂CO₃:
Ksp = [Ag+]²[CO₃²⁻]
Let x be the molar solubility of Ag₂CO₃. Then, [Ag+] = 2x and [CO₃²⁻] = x. Substituting into the Ksp expression and solving for x:
8.1 x 10⁻¹² = (2x)² * x
x = 1.2 x 10⁻⁴ M
To convert to g/L, we need to multiply by the molar mass of Ag2CO3 (331.8 g/mol):
solubility = 1.2 x 10⁻⁴ * 331.8 = 0.0398 g/L
(c) Bi₂S₃:
Ksp = [Bi³⁺]²[S²⁻]³
Let x be the molar solubility of Bi₂S₃. Then, [Bi3+] = 2x and [S2-] = 3x. Substituting into the Ksp expression and solving for x:
1.6 x 10⁻⁷² = (2x)²*(3x)³
x = 3.2 x 10⁻¹⁶
To convert to g/L, we need to multiply by the molar mass of Bi₂S₃ (514.2 g/mol):
solubility = 3.2 x 10⁻¹⁶ * 514.2 = 1.65 x 10⁻¹³ g/L
In summary, using the suitable Ksp formula and solving for the unknown variable, we can compute the molar solubility and solubility in g/L of salt at a particular temperature. The molar solubility is represented in M units, but the solubility in g/L is calculated by multiplying the molar solubility by the salt's molar mass. The Ksp value indicates the salt's dissolving equilibrium constant and gives information on the relative solubility of various salts under the same circumstances.
Learn more about Molar Solubility:
https://brainly.com/question/28202068
#SPJ4
During the SI Scavenger Hunt Lab, a student measured her foot length using a meter stick and recorded it as 25 cm. However, at the shoe store, the sales staff measured the length of her foot to be 23.7 cm. If the shoe store’s measurement is accepted as correct, what is the percent error of her meter stick measurement?
Answers
Answer:
Percent error in meter stick measurement is 5.5%.
Explanation:
Given data:
Measured value = 25 cm
Accepted value = 23.7 cm
percent error = ?
Solution:
Subtract the accepted value from measured value divided by accepted value multiply by 100.
Formula:
(measured value - accepted value / accepted value ) × 100
Now we will put the values.
(25 cm - 23.7 cm/23.7 cm ) × 100
1.3 cm/23.7 cm ) × 100
0.055× 100
5.5%
Percent error in meter stick measurement is 5.5%.
How does an emerging idea differ from scientific consensus? Which best describes emerging scientific ideas?
Answers
Emerging scientific ideas are new theories or ideas that are gaining attention in the scientific community, but have not yet been fully accepted or confirmed.
Emerging ideas refer to the new and innovative ideas or theories that have yet to gain full scientific acceptance. While a scientific consensus is a view or theory that has been universally accepted and confirmed by multiple experiments or research, an emerging scientific idea is a new and unproven theory or idea that is gaining attention in the scientific community. These emerging ideas may also be referred to as scientific hypotheses. In contrast to scientific consensus, emerging scientific ideas have not yet been subjected to rigorous testing and confirmation.
They are generally proposed to explain new observations or experimental results, which have not yet been fully understood or explained by established scientific theories. Emerging scientific ideas can have the potential to challenge the current scientific consensus. If an emerging scientific idea is found to be valid, it can ultimately lead to the establishment of a new scientific consensus. For example, the emerging scientific idea of the Higgs boson particle led to the discovery of a new field in particle physics, which is now an established scientific consensus.
for such more questions on scientific
https://brainly.com/question/29886197
#SPJ8
why is time an independent variable
Answers
in which type of condition is a conditions is a landslide most likely to occur

Answers
Answer:
B. In a wet, hilly area
Explanation:
We know that for a landslide to happen, there has to be a down hill slope so it can fall.
This eliminates answers C, D and E.
So we are left with A and B
Now we can look at this in a real life scenario. If you have ever walking on wet ground, you will know that is weaker than dry ground. Knowing this, we know the answer will have to be answer B.
A gas has a pressure of 2.36 kPa at 62 °C. What is the pressure at standard
temperature?
Answers
Answer:
P2 = 1.94 kPa
Explanation:
Given the following data;
Initial pressure = 2.36 kPa
Initial temperature = 62°C
Standard temperature = 0°C
Conversion:
Kelvin = 273 + C
Kelvin = 273 + 62 = 335 K
Kelvin = 273 + 0 = 273 K
To find the final pressure, we would use Gay Lussac's law;
Gay Lussac states that when the volume of an ideal gas is kept constant, the pressure of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Gay Lussac's law is given by;
\( PT = K\)
\( \frac{P1}{T1} = \frac{P2}{T2}\)
Making P2 as the subject formula, we have;
\( P_{2}= \frac{P1}{T1} * T_{2}\)
\( P_{2}= \frac{2.36}{335} * 273 \)
\( P_{2}= 0.0071 * 273 \)
P2 = 1.94 kPa
what causes potassium ions to leave the axon just after the peak of the action potential?
Answers
The cause of potassium ions leaving the axon just after the peak of the action potential is due to the opening of voltage-gated potassium channels. These channels open in response to the depolarization that occurs during the action potential.
And also during an action potential, depolarization causes voltage-gated sodium channels to open, allowing sodium ions to flow into the axon. This influx of positive ions causes the membrane potential to rapidly rise and eventually reach a peak. At this point, voltage-gated potassium channels begin to open, allowing potassium ions to flow out of the axon. This outflow of positive ions causes the membrane potential to rapidly repolarize and eventually reach a hyperpolarized state. This rapid efflux of potassium ions is what causes them to leave the axon just after the peak of the action potential.
When the membrane potential reaches its peak, the voltage-gated potassium channels open, allowing potassium ions to flow out of the axon, leading to repolarization and the eventual return of the membrane potential to its resting state.
To know more about depolarization :
https://brainly.com/question/29698882
#SPJ11
PHOTO ABOVE
Answer Choices:
-Sp
-Sp2
-Sp3
-Sp4
I don’t get this!

Answers
Answer:
By just counting sigma bond and lone pair in central atom , one can easily find hybridization.
CO2 sp
SO2 sp2
NH3 sp3
BCl3 sp2
I Need help with this

Answers
In the given reaction ₉₆²⁴⁶Cm + ₆¹²C ---> 4 ¹on + X it shows an example of an artificial transmutation reaction.
An artificial transmutation reaction may resemble this. The method of causing nuclear reactions by blasting atomic nuclei with high-energy particles like ions or neutrons is referred to as artificial transmutation.
In this instance, the transmutation is induced by bombarding the carbon nucleus (C) with additional particles or a high-energy beam, resulting in the production of the following products: Element X and 4 1on (Helium-4)
Blasting an element with a basic particle, an element can be artificially transmuted into a different element.
Learn more about artificial transmutation, here:
https://brainly.com/question/2472288
#SPJ1
What is the empirical formula of a compound containing 5.03 grams carbon, 0.42 grams hydrogen, and 44.5 grams chlorine?
a. c2h5ci
b. c2h2c1
c. chcia
d. chci
Answers
The empirical formula of the compound is CHCl₃.
Calculation:Given,
Mass of carbon = 5.03 g
Mass of hydrogen = 0.42 g
Mass of chlorine = 44.5 g
Molecular weight of carbon = 12 g
Molecular weight of hydrogen = 1 g
Molecular weight of chlorine = 35.4 g
First, calculate the moles of each element,
Moles = given mass/ molecular weight
Moles of carbon = 5.03/12 = 0.42
Moles of hydrogen = 0.42/1 = 0.42
Moles of chlorine = 44.5/ 35.4 = 1.26
Divide the moles of each element by the smallest number of moles,
0.42 mol of C/ 0.42 = 1 C
0.42 mol of H/ 0.42 = 1 H
1.26 mol of Cl/0.42 = 3 Cl
The ratio of elements is 1:1:3
Therefore the empirical formula of the compound will be CHCl₃.
Learn more about empirical formula here:
https://brainly.com/question/20708102
#SPJ4
Answer:CHCl₃
Explanation:
silver chloride contains 56.34 % ag by mass. calculate the mass (in kg) of silver chloride required to plate 165 micrograms of ag.
Answers
The mass of silver chloride (AgCl) required is 390mg when it contains 56.34 % ag by mass and 165 micrograms of Ag.
Given silver chloride contains (p1) = 56.34 % ag by mass
Then, 1 mole of AgCl contains 0.5634 moles of Ag
1 mole of Ag is obtained from 1/0.5634 mole of AgCl
The mass of silver (Ag) = m = 165mg = 165 x 10^-6g
The atomic mass of silver (M) = 107g/mole
Then the number of moles of Ag required (n) = 165/107 = 0.00154mole
Now 0.00154 mole of Ag is obtained from = 0.00154/0.5634 = 0.00273 moles of AgCl.
The molar mass of AgCl = 143g/mole
mass of AgCl required = moles x molar mass = 0.00273 x 143 = 0.390g
Hence the mass of AgCl required is 390mg
To learn more about AgCl click here https://brainly.com/question/17102479
#SPJ4
The number of unpaired electrons in a nitrogen atom is
Answers
Answer:
3
Explanation:
A nitrogen atom has 3 unpaired electrons
The plant on the left is growing more because it has been receiving more water
Observation or inference
Answers
Answer:
am not sure i understand but i think it got more chlorophyll
5.2 kg of argon fills an insulated, rigid container which has a volume of 0.8 . if the temperature within the container is 83 , what is the pressure of the argon in kpa?
Answers
We can solve the problem using the Ideal Gas Law which states that:
PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant and T is the temperature.
Rearranging this equation, we get:
P = nRT/V.
We have to find the pressure of argon in kPa given that it fills an insulated, rigid container with a volume of 0.8 m3 and the temperature within the container is 83°C. The number of moles can be calculated as:
n = mass/molar mass = 5.2 kg/39.948 g/mol = 130.22 moles.
The gas constant R is equal to 8.314 J/(mol K).
The temperature has to be in Kelvin, which is equal to= 83°C + 273.15 = 356.15 K.
Therefore, the pressure can be calculated.
The pressure of the argon in kPa is 3696.98
To know more about Gas Law visit:
brainly.com/question/30458409
#SPJ11