Although both sugar and salt are soluble in water, the way in which they dissolve is different. explain how their dissolving process is different, and elaborate why these differences exist.
Answers
Salt starts off as a compound, as it dissolves in ions, which always happens. Sugar, known as covalent, is bonded because they share electrons and stays together in compounds.
How do ionic compounds dissolve in water?
Ionic compounds dissolve in polar solvents, especially water. this happens when the positive cation from the ionic solid is attracted to the negative end of the water molecule (oxygen) and the negative anion of the ionic solid is attracted to the positive end of the water molecule (hydrogen)
Why do salt and sugar dissolve differently in water?
The polar water molecules attract the oppositely charged polar areas of the sucrose molecules and pull them away, leading to dissolving. Since the ions in salt and therefore the molecules bin sugar are very different, their solubilities tend to vary .
Learn more about covalent compounds :
brainly.com/question/11651796
#SPJ4
Related Questions
when salt is dissolved in water the result is a
Answers
Answer:
Water molecules pull the sodium and chloride ions apart
Explanation:
why fuse wire is made of an alloy of lead and tin?
Answers
Hope it helps
If it does could I get bra
resonance can only occur when the elements in the molecule keep the same formal charge.True False
Answers
False. Resonance occurs when electrons in a molecule are delocalized and can move freely between different atoms, leading to multiple possible structures.
A key idea in organic chemistry is, resonance helps to explain why molecules are stable and reactive. Electrons are distributed over a number of atoms in a resonant structure as opposed to being concentrated on just one. Since the electrons are less likely to oppose one another, the entire structure may become more stable as a result.
Although formal charges can be used to forecast how electrons would be distributed within a molecule, resonance is not always dependent on them. The formal charges of some resonant structures may even differ from those of the original structure. In order to completely grasp the behavior of organic molecules, it is crucial to appreciate the concepts of resonance and electron delocalization.
Learn more about Electrons
brainly.com/question/1255220
#SPJ4
Which combination of reagents is the least effective in generating sodium ethoxide, CH3CH2ONa?
Answers
The least effective combination of reagents in generating sodium ethoxide is using NaOH instead of Na as the reducing agent. NaOH is a weaker reducing agent, leading to a slower reaction and a lower yield of sodium ethoxide.
In order to generate sodium ethoxide, we need to combine ethanol with sodium metal. However, not all combinations of reagents are equally effective in generating sodium ethoxide.
One combination of reagents that is least effective in generating sodium ethoxide is using sodium hydroxide (NaOH) instead of sodium metal (Na). Sodium hydroxide is a weaker reducing agent compared to sodium metal, meaning it is less effective in donating electrons to ethanol to form the sodium ethoxide. Additionally, the reaction between NaOH and ethanol is slower compared to that of Na and ethanol. This means that even if we use excess NaOH, the reaction will not proceed to completion, resulting in a lower yield of sodium ethoxide.
In summary, the least effective combination of reagents in generating sodium ethoxide is using NaOH instead of Na as the reducing agent. NaOH is a weaker reducing agent, leading to a slower reaction and a lower yield of sodium ethoxide.
To know more about sodium ethoxide visit :
https://brainly.com/question/31993479
#SPJ11
what is the function of glycerin in this experiment?
Answers
a compound is found to be 64.9% carbon, 13.5% hydrogen and 21.6% oxygen. if its molecular mass is 74 g/mol, the molecular formula is
Answers
The molecular formula is C32H80O8.
What is empirical formula?The empirical formula is the simplest whole-number ratio of the atoms of different elements in a compound. It is determined by experiment and is not necessarily the same as the molecular formula. The empirical formula does not provide information about the arrangement of atoms in the molecule or any information about the isomers of the molecule. The empirical formula of a compound is also referred to as its simplest formula or simplest ratio.
The molecular formula for this compound can be determined by first calculating the empirical formula.
The empirical formula can be determined by dividing the mass percentage of each element by its relative atomic mass.
Carbon: 64.9/12 = 5.41
Hydrogen: 13.5/1 = 13.5
Oxygen: 21.6/16 = 1.35
Now, to get the empirical formula, we divide each number by the smallest one (1.35):
Carbon: 5.41/1.35 = 4
Hydrogen: 13.5/1.35 = 10
Oxygen: 1.35/1.35 = 1
Therefore, the empirical formula is C4H10O.
The molecular formula is then determined by multiplying the subscripts of the empirical formula by the given molecular mass (74 g/mol). The molecular mass must be divisible by the empirical formula mass (which is 74 g/mol).
C4H10O * 8 = C32H80O8
The molecular formula is C32H80O8.
To learn more about empirical formula
https://brainly.com/question/1603500
#SPJ4
Which of the following processes does our Sun and other stars in our galaxy use to create energy?
Answers
Answer:
fusion reaction
The simple answer is that the sun, like all stars, is able to create energy because it is essentially a massive fusion reaction. Scientists believe that this began when a huge cloud of gas and particles (i.e. a nebula) collapsed under the force of its own gravity – which is known as Nebula Theory.
Explanation:
hope this helps
Humans become part of the carbon cycle when they
A. breathe in carbon dioxide,
B. release carbon as a waste product,
C. produce new carbon compounds in their cells,
Answers
Answer:
Human activities have a tremendous impact on the carbon cycle
Explanation:
Burning fossil fuels, changing land use, and using limestone to make concrete all transfer significant quantities of carbon into the atmosphere. ... The ocean absorbs much of the carbon dioxide that is released from burning fossil fuels.
Dinitrogen pentoxide is used in the preparation of explosives. If 7.93 mol of
dinitrogen pentoxide undergoes simple decomposition, what volume of Oz(9) is
produced at 48.0 °C and 125 kPa?
Answers
The volume of O₂ produced: 84.6 L
Further explanationGiven
7.93 mol of dinitrogen pentoxide
T = 48 + 273 = 321 K
P = 125 kPa = 1,23365 atm
Required
Volume of O₂
Solution
Decomposition reaction of dinitrogen pentoxide
2N₂O₅(g)→4NO₂(g)+O₂ (g)
From the equation, mol ratio N₂O₅ : O₂ = 2 : 1, so mol O₂ :
= 0.5 x mol N₂O₅
= 0.5 x 7.93
= 3.965 moles
The volume of O₂ :
\(\tt V=\dfrac{nRT}{P}\\\\V=\dfrac{3.965\times 0.082\times 321}{1.23365}\\\\V=84.6~L\)
identify the limiting reactant when 7.28 grams of magnesium oxide reacts with 4.50 grams of aluminum to make magnesium and aluminum oxide? i need a typed answer a link wont work
Answers
Answer:
the limiting reactant is aluminum
Explanation:
the two main human derived sources of carbon dioxide are
Answers
The two main human-derived sources of carbon dioxide are: 1. Burning of fossil fuels, 2. Deforestation and land-use changes.
Burning of fossil fuels: The combustion of fossil fuels such as coal, oil, and natural gas for energy production, transportation, and industrial processes releases significant amounts of carbon dioxide into the atmosphere. This is a major contributor to the increase in atmospheric carbon dioxide levels and the greenhouse effect.
Deforestation and land-use changes: The conversion of forests and other natural ecosystems into agricultural lands or urban areas results in the release of carbon dioxide. Trees and vegetation act as carbon sinks, absorbing carbon dioxide during photosynthesis. When forests are cleared or destroyed, this stored carbon is released back into the atmosphere as carbon dioxide through processes like burning and decomposition.
These two sources contribute significantly to the anthropogenic increase in carbon dioxide levels, which has implications for climate change and global warming.
To know more about carbon dioxide, refer here:
https://brainly.com/question/1457431#
#SPJ11
under which conditions do you expect helium gas to deviate most from ideal behavior?
Answers
Answer:
Explanation:
[2-0] NC Special Operation
[2] Supervisor
[2-1] Emergency Response
[2-2] Juggernaut
[2-3] Commander
[2-4] Division Director
Which of the following multistep reaction pathways will give a higher yield and why? Pathway #1, because there is much less steric hindrance for primary substrates than for secondary substrates in the SN2 reaction, which is the first mechanism in each of the two pathways. Pathway #2, because there is much steric hindrance for secondary substrates than for primary substrates in the SN2 reaction, which is the first mechanism in each of the pathways. Both pathways will give the same yield.
Answers
Pathway #1 will give a higher yield because there is much less steric hindrance for primary substrates than for secondary substrates in the SN2 reaction, which is the first mechanism in each of the two pathways.
The SN2 reaction proceeds via a concerted, bimolecular, one-step mechanism, where the nucleophile attacks the electrophilic carbon center, and the leaving group is simultaneously displaced. In pathway #1, the SN2 reaction involves a primary substrate with a less hindered leaving group, which will react faster and give a higher yield.
On the other hand, pathway #2 involves a secondary substrate with a more hindered leaving group, which will react slower and give a lower yield. Hence, pathway #1 is more efficient because of the higher reactivity of the primary substrate and the less steric hindrance in the reaction mechanism.
Therefore, the pathway #1 will give a higher yield than pathway #2 in a multistep reaction pathway because there is much less steric hindrance for primary substrates than for secondary substrates in the SN2 reaction, which is the first mechanism in each of the two pathways.
Learn more about leaving group here:
https://brainly.com/question/31831803
#SPJ11
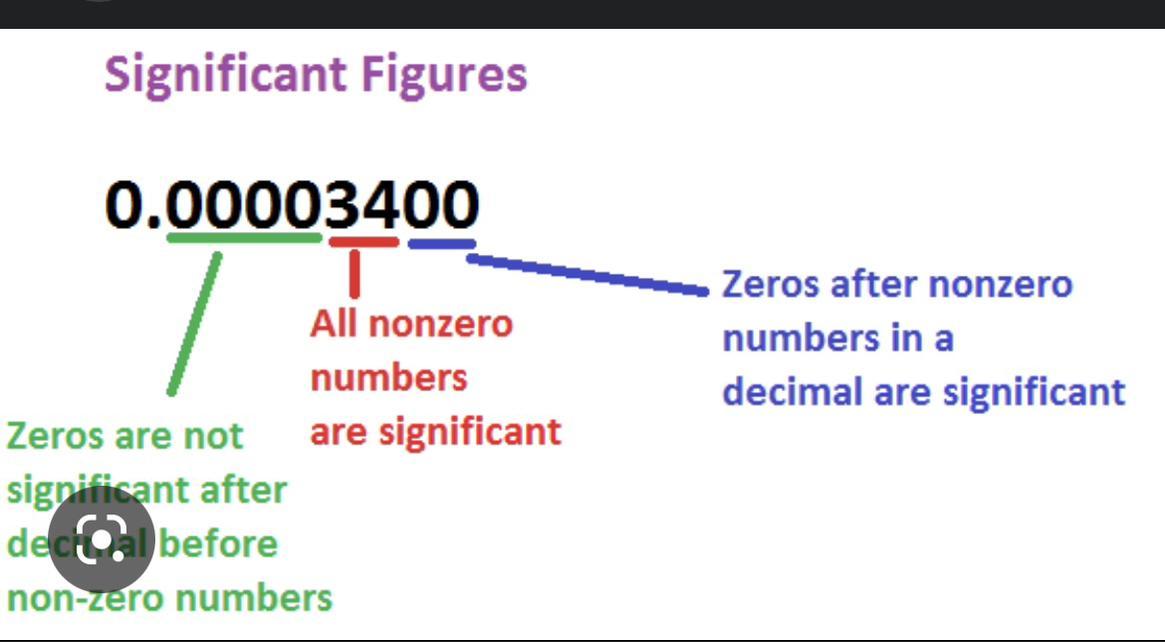
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
the formula mass of an ionic compound is the sum of the masses of the atoms contained in one
Answers
Formula unit of the compound. The formula mass of an ionic compound can also be referred to as the formula weight.
To calculate the formula mass of an ionic compound, you need to know the chemical formula of the compound and the atomic masses of the elements that make up the compound.
Here's an example:
What is the formula mass of calcium chloride, CaCl2?
Step 1: Find the atomic mass of each element
Calcium (Ca) has an atomic mass of 40.08 g/molChlorine (Cl) has an atomic mass of 35.45 g/molStep 2: Determine the number of atoms of each element in the formula unit
There is one calcium atom (Ca) and two chlorine atoms (Cl) in each formula unit of calcium chloride, CaCl2Step 3: Calculate the formula mass
To calculate the formula mass, you multiply the atomic mass of each element by the number of atoms of that element in the formula unit, then add the results together:Formula mass = (1 x 40.08 g/mol) + (2 x 35.45 g/mol)
Formula mass = 110.98 g/mol
Therefore, the formula mass (or formula weight) of calcium chloride,
CaCl2, is 110.98 g/mol.
To learn more about compound refer to this link
https://brainly.com/question/19458442
#SPJ4
What is a quantum of light energy called?
O A. A photon
OB. A frequency
O C. A proton
O D. An electron
Answers
Answer:
A) photon
Explanation:
hope it helps and give brainliest if its correct
Answer:
PhotonExplanation:
please mark brainliest pleaseHow old is the bedrock in massena
Answers
describe the temperature, moisture and air pressure associated with a continental polar air mass.
Answers
The variance in the US continental region is brought on by the shift in daytime and nighttime weather patterns.
Continental polar air mass -Cold, dry, and stable air masses are found in the continental polar (cP) or continental arctic (cA) regions. Radiative cooling causes these air masses to form over northern Canada and Alaska. They travel south, then east via the Plains and the Rockies.
During the winter, a continental polar air mass can develop over the land. It comes from northern Canada or Alaska in the Northern Hemisphere. It transports dry weather to the United States as it goes south. Low humidity and temperature are both present.
These factors contributed to the polar air mass:
Breezeextreme humiditythe evening's low temperatureDuring the colder months of the year, continental polar air typically forms over vast land masses.
A cool breeze blows across the upper section of the area, while a warm breeze blows through the lower part.
To learn more about continental polar air mass from given link
https://brainly.com/question/22790966
#SPJ4
1.) You have a sample of 1.64 moles of aluminum carbonate is mixed with lithium to produce lithium carbonate and aluminum. How many moles of lithium would completely react with all the aluminum carbonate.
2.) a synthesis reaction between magnesium and nitrogen forms an ionic compound magnesium nitride. If you have 4.226 moles of magnesium how many grams of nitrogen will completely react with the magnesium.
3.) given the following equation 8Fe+S8>8FeS, how many grams FeS are produced, and what mass of iron is needed to react with 16 grams of sulfur
4.) B2H6+3O2>HBO2+2H2O, what mass of O2 will be needed to burn 31.6g B2H6, and how many miles of water are produced from 12.8g B2H6
Answers
Lithium carbonate contains 18.78% mass percent lithium. It should be noted that a compound's overall percentage composition of all its constituent elements is always 100%.
Is lithium carbonate a depressive medication?Only depression related to bipolar illness is authorized for lithium use. When combined with an antidepressant, it may also be successful in alleviating other types of depression, although further research is required. Discuss the possibility of adding lithium with your doctor if you are on an antidepressant but are still experiencing symptoms.
What purpose does lithium carbonate serve?This substance is employed for the treatment of mania and depression (bipolar disorder). By bringing certain natural compounds back into equilibrium in the brain, it helps to calm mood and lessen excessive behavior.
To know more about Lithium Carbonate visit:
https://brainly.com/question/15127937
#SPJ1
Which reaction takes place in a nuclear fission reactor?
OCH N
→
239
O Pu+ He 242 Cm
94
96
O 27 Co+He→Co+n
139
O 235 U+n Kr+¹Ba+3/n
56
92

Answers
The reaction that takes place in a nuclear fission reactor is as follows: 235/92 U + 1/0n 94/36Kr + 139/56 Ba + 3/0n.
What is a nuclear fission reactor?A nuclear fission reactor is the place where nuclear chain reactions occur that produce energy by fission.
Nuclear fission is the nuclear reaction in which a large nucleus splits into smaller ones with the simultaneous release of energy.
Therefore, the option that involves the splitting of atoms into smaller ones is as follows: 235/92 U + 1/0n 94/36Kr + 139/56 Ba + 3/0n.
Learn more about nuclear fission reactor at: https://brainly.com/question/10203508
#SPJ1
what is the specific heat of iron if 985 J of energy is required to raise the temperature of 158.5 g from 10.0°C to 24.0° C ?
Answers
Answer: 0.44
Explanation:

The specific heat of iron if 985 J of energy is required to raise the temperature of 158.5 g from 10.0°C to 24.0°C is 0.44J/g°C.
How to calculate specific heat capacity?The specific heat capacity of a substance can be calculated using the following formula:
Q = m × c × ∆T
Where;
Q = quantity of heat absorbed or releasedm = mass of the substancec = specific heat capacity∆T = change in temperature985 = 158.5 × c × (24°C - 10°C)
985 = 2219c
c = 985/2219
c = 0.44 J/g°C
Therefore, the specific heat of iron if 985 J of energy is required to raise the temperature of 158.5 g from 10.0°C to 24.0°C is 0.44J/g°C.
Learn more about specific heat capacity at: https://brainly.com/question/1747943
#SPJ2
Which biotic factor would have the greatest impact on the number of rabbits in a meadow?
Answers
The biotic factor that would have the greatest impact on the number of rabbits in a meadow is the availability of food. Food availability directly affects the survival, reproduction, and overall population size of rabbits.
Rabbits are herbivores, and their diet consists mainly of plant materials such as grasses, herbs, and other vegetation found in meadows. The abundance and quality of food sources in the meadow will determine the carrying capacity of the habitat for rabbits.
If there is an ample supply of food in the meadow, the rabbit population can thrive and increase in number. Sufficient food resources provide the necessary energy and nutrients for rabbits to survive, reproduce, and raise their offspring. In such cases, the rabbit population can grow and reach its maximum potential.
On the other hand, if the food supply is limited or becomes scarce, it will have a significant impact on the rabbit population. Insufficient food availability can lead to malnutrition, decreased reproductive success, and increased vulnerability to predation and diseases. As a result, the rabbit population may decline, and individuals may struggle to survive.
Therefore, the availability of food is a critical biotic factor that directly influences the number of rabbits in a meadow.
To know more about biotic factor click this link -
brainly.com/question/29776080
#SPJ11
Which postulate of the kinetic-molecular theory applies to the particles of a liquid?
They do not move freely but vibrate in a stationary position.
They can be compressed, which causes a large change in volume.
They have so much kinetic energy that their intermolecular forces are negligible.
They have enough kinetic energy to easily slide by each other.
Answers
The particles of liquid have enough kinetic energy to easily slide by each other. The correct option is D.
What is kinetic-molecular theory?The kinetic-molecular hypothesis stated matter states and is based on the assumption that matter is made up of tiny particulate that are constantly in motion.
This theory adds to the knowledge of observable properties and behaviors of solids, liquids, and gases.
Because they are densely packed and vibrate in place, solids have the lowest kinetic energy.
Since liquids have higher kinetic energy, the particles slide past each other. Because gases have the most kinetic energy, they float around in the air.
Particles in liquids are very close together and move randomly throughout the container.
Due to the shorter distances between particles, particles move rapidly in all orientations but strike with each other more frequently than in gases.
Thus, the correct option is D.
For more details regarding kinetic molecular theory, visit:
https://brainly.com/question/15013597
#SPJ6
NaHCO3 + HC2H3O2 → NaC2H3O2 + H2O + CO2
What are the reactants and products also add why
MARKING BRAINLIEST
Answers
Choose the products that complete the reaction. The chemical equation may not be balanced.
NaHCO3 + HC2H3O2
Answer: NaC2H3O2 + CO2 + H2O
which of the following pairs are ionic compounds ( Al,Cl),(Na,O),(Al,F)
Answers
Answer:
This answer I think it is Al,F
Name two characteristics that a thing must have to be called matter.
Answers
Answer:
mass is one
Explanation:
Answer:
mass and volume are the correct answers
What state had the fewest farms? Wisconsin Georgia Illinois
Answers
2. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. would the titration have required more, less or the same amount of naoh solution for a complete reaction? explain.
Answers
The titration would require the same amount of NaOH.
Number of moles and dilutionThe number of moles of solute in a solution remains the same irrespective of the amount of water added to dilute the solution.
This is in accordance with the dilution law which says that the number of moles of a solution before dilution is the same as after dilution.
Thus, the amount of NaOH needed to neutralize the vinegar will remain the same for a complete reaction since the number of moles of solute in the vinegar has not changed.
More on number of moles can be found here: https://brainly.com/question/14919968
#SPJ1
select correct examples of linear molecules for two electron groups. select correct examples of linear molecules for two electron groups. xef2 cs2 h2o h2s
Answers
The correct examples of linear molecules for two electron groups are: XeF2 and CS2.
Linear molecules are molecules that have a straight line shape. These molecules are comprised of a central atom that is surrounded by two or more other atoms or groups of atoms. Linear molecules are a kind of molecular geometry, which explains the shape of a molecule based on the positions of its atoms.
Examples of linear molecules for two electron groups are:
Xenon difluoride (XeF2) is a colorless gas that has a linear shape. The molecule is made up of a central xenon atom that is bonded to two fluorine atoms.
Carbon disulfide (CS2) is a colorless liquid that is also linear. The molecule is composed of a central carbon atom that is bonded to two sulfur atoms.
Hence, the correct examples of linear molecules for two-electron groups are XeF2 and CS2.
To know more about Linear molecules, refer here:
https://brainly.com/question/16770400#
#SPJ11
You add 2000 J of heat to a piece of iron and you observe a temperature rise of 20.0 ∘C. What is the mass of the iron? How much heat would you have to add to an equal mass of water to get the same temperature rise? Explain the difference in your results for Parts A and B
Answers
To determine the mass of the iron, we can use the heat capacity equation: Q = mcΔT Where Q is the heat energy, m is the mass, c is the specific heat capacity, and ΔT is the temperature change.
In this case, we have Q = 2000 J and ΔT = 20.0 °C. The specific heat capacity of iron is approximately 450 J/(kg·°C).
Rearranging the equation to solve for mass:
m = Q / (cΔT)m = 2000 J / (450 J/(kg·°C) × 20.0 °C)m ≈ 0.222 kg
Therefore, the mass of the iron is approximately 0.222 kg. To determine the amount of heat required to raise an equal mass of water by the same temperature, we can use the same heat capacity equation. The specific heat capacity of water is approximately 4186 J/(kg·°C).
Using the same equation, but with the mass of water being 0.222 kg and ΔT = 20.0 °C, we can calculate the heat energy:
Q_water = (0.222 kg) × (4186 J/(kg·°C)) × (20.0 °C)
Q_water ≈ 1787 J
Therefore, to achieve the same temperature rise, you would need to add approximately 1787 J of heat to an equal mass of water.The difference in results between the mass of the iron and the heat required for water is due to the different specific heat capacities of the substances. The specific heat capacity represents the amount of heat energy required to raise the temperature of a given mass of a substance by 1 degree Celsius. Since the specific heat capacity of water is significantly higher than that of iron, a greater amount of heat energy is required to achieve the same temperature rise in water compared to iron, even if the masses are equal.
To know more about heat capacity, click here https://brainly.com/question/28302909
#SPJ11