Answers
Cells primarily use sugars, such as glucose, for energy production through a process called cellular respiration.
What are cells?Cells are the basic building blocks of life. They are the smallest unit of life that can carry out all the functions necessary for an organism to survive and reproduce. Cells are found in all living organisms, from simple one-celled organisms like bacteria, to complex multicellular organisms like plants and animals.
Glucose is broken down in the presence of oxygen to produce ATP (adenosine triphosphate), which is the energy currency of the cell. While fats and proteins can also be used for energy production, glucose is the preferred energy source for most cells in the body. Minerals, on the other hand, are not a direct source of energy for cells but are essential for many cellular processes and functions.
Learn about cells here https://brainly.com/question/13920046
#SPJ1
Related Questions
Please help I will give brainiest

Answers
Answer:
i agree with imj, smaller computers have smaller batteries, but i also think that circut boards is the true answer, circut boards run the computer but batteries power it i think its *Circut Board*
When fossil fuels are burned, they emit carbon dioxide into the atmosphere. After centuries of large amounts of carbon dioxide accumulating in the atmosphere, the earth's temperature increases by 1°C.
What is the connection between increasing carbon dioxide and increasing temperature?

Answers
The connection between increasing carbon dioxide and increasing temperature is: carbon dioxide absorbs heat from the sun and traps it in earth's atmosphere. Since the heat cannot escape, it causes the earth's temperature to increase which is the first option.
When carbon dioxide (CO₂) and other greenhouse gases are present in the atmosphere, they act as a natural blanket, allowing sunlight (solar radiation) to pass through and reach the Earth's surface. Some of this solar radiation is absorbed by the Earth's surface, while the rest is reflected back towards space as heat (infrared radiation). However, greenhouse gases like carbon dioxide have the property of absorbing and re-emitting infrared radiation.
Learn more about fossil fuel here
https://brainly.com/question/2029072
#SPJ1
0.2g of sand in two-third in liter of ethanol . What is the concentration in g per dm cube
Answers
The mass concentration of sand in the ethanol solution is 0.299 g/dm³.
What is the concentration in grams per dm³?To find the concentration in grams per cubic decimeter (g/dm³), we first need to convert the volume from liters to cubic decimeters (dm³). Since 1 liter is equal to 1 cubic decimeter, we can directly convert the volume.
Given:
Mass of sand = 0.2 g
Volume of ethanol = two-thirds liter
Converting volume to dm³:
1 liter = 1 cubic decimeter
two-thirds liter = (2/3) cubic decimeter = 0.67 dm³ (rounded to two decimal places)
Now we can calculate the concentration in g/dm³ by dividing the mass of sand by the volume in dm³:
Concentration = Mass / Volume
Concentration = 0.2 g / 0.67 dm³
Concentration ≈ 0.299 g/dm³ (rounded to three decimal places)
Learn more about mass concentration at: https://brainly.com/question/23437000
#SPJ1
Calculate the mass of a body
Whose volume is
Is 2cm3 and
density is 520cm3
Answers
Answer:
The answer is
1040gExplanation:
Density = mass / volume
mass = density × volume
volume = 2cm³
density = 520g/cm³
mass = 2 × 520
= 1040g
Hope this helps you
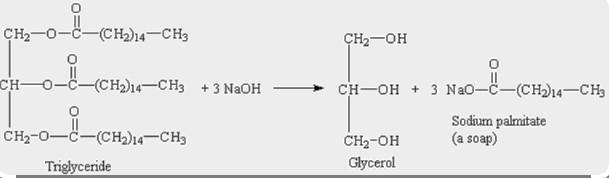
1) write a balanced equation to show the hydrolysis of glycerol tristearate (tristearin, a triple ester of glycerol) using water and sodium hydroxide to make soap. in addition to the molar ratios, give the mass ratios, i.e., the ratios of masses that react according to the reaction stoichiometry) based on 20 g of tristearin.
Answers
Mass ratio of Tristearin that react according to the reaction : \(NaOH\) is
20 : 27
The main fat in beef is tristearin. A molecule of glycerine that has interacted with three (3) molecules of the fatty acid stearic acid is known as a triglyceride.
1 mole of Tristearin requires 3 moles of \(NaOH\) to react to give 1 mole of glycerol & 3 moles of sodium sterate.
Molar ratio of reactant Tristearin : \(NaOH\) = 1 : 3
Molar weight of Tristearin = 891.5 g/mol
=> 20 g Tristearin = \(\frac{20}{891.5} moles\)
=> \(NaOH\) required to react with \(\frac{20}{891.5}\) moles of Tristearin = \(3\times\frac{20}{891.5} moles\) = 0.0673 moles
=> Molecular weight of \(NaOH\) = 40 g/mol
=> \(NaOH\) mass required to react with 20 g Tristearin = 0.0673 × 40g = 2.7g
∴ Mass ratio of Tristearin: \(NaOH\) is 20 : 27.
Learn more about Tristearin:
brainly.com/question/7175814
#SPJ4
A series of measurements in the lab led to an experimental result of 32.9 mL, with a calculated standard deviation of 0.3 mL. What is the standard way to report this result?
Answers
Answer: The standard way to report this result is \(32.9\pm 0.3 mL\)
Explanation:
The standard method of representing a result is:
\(\text{Calculated value }\pm \text{ Standard deviation}\)
The reporting of a result is done in correct significant figures.
We are given:
Calculated value = 32.9 mL
Standard deviation = 0.3 mL
Rule of significant figures applied when numbers are added or subtracted:
The number having less number of significant figures after the decimal point will determine the number of significant figures in the final answer.
Number of significant figures after the decimal point = 1
Hence, the standard way to report this result is \(32.9\pm 0.3 mL\)
PLSSSSSSSSS HELP!!!!!!!! FIRST TO AWNSER GETS BRAINLIEST!!!!!!!!
Research four illnesses that afflict humans. Each illness should be caused by a pathogenic organism of a different type:
an illness caused by a bacterium
an illness caused by a virus
an illness caused by a fungus
an illness caused by a protist
Answers
Answer:
measles are caused by virus
strep throat is caused by bacterium
aspergillosis caused by fungus
malaria caused by protist
hope this helps!
Explanation:
helppppppp! why should i use spr??

Answers
SPR can be used in order to do real time monitoring and to evaluate the advancement of compounds.
Why should we use SPR?SPR provide information to evaluate whether or not compounds should advance to the next stage of investigation. It also provides real-time monitoring.
So we can conclude that SPR can be used in order to do real time monitoring and to evaluate the advancement of compounds.
Learn more about monitoring here: https://brainly.com/question/13163394
#SPJ1
Using the table of average bond energies, determine the total bond energy for the products in the combustion of ethene: C2H4 + 3 O2 --> 2 CO2 + 2 H2O

Answers
Answer:
\(B\text{ : }5064\text{ KJ/mol}\)Explanation:
Here, we want to get the total bond energy for the products
On the products side, we have 4 C=O bonds formed and 4 O-H bonds formed
We have the values from the given table above
Thus, we have the energy as the sum of the energy of the bonds
Mathematically, we have that as:
\((4\times\text{ 799 \rparen + \lparen4 }\times467)\text{ = 5,064 KJ/mol}\)We would be having the answer as negative since the process of bond formation is exothermic (heat is given off and enthalpy change value is negative)
If the mass of a stick of butter________ then no mass was created when it melted
Answers
Answer:
decreases
Explanation:
Melting is the process of turning solid materials into liquids by introducing heat. If the mass of a stick of butter decreases then no mass was created when it melted.
Melting is the process of a solid changing from a solid to a liquid when heated to a specific temperature. The set temperature at which a solid begins to transform into a liquid is known as the melting point.
A substance will change from a solid to a liquid when the temperature is raised to its melting point. since of this, the substance's volume will grow since its molecules will be spaced more apart.
To know more about melting, visit;
https://brainly.com/question/34580322
#SPJ7
Putting your ear to a wall will allows you to hear the noise the other side better than through the air. (True or False) Explain?
Answers
Based on the information given, it should be noted that sound travels through air, and putting your ear to a wall will not allow the person to hear the noise on the other side better. Therefore, it is false.
How does sound travel?It should be noted that when sound is created, the air particles vibrate and collide with each other. The vibrating particles pass the sound through to a person's ear and then vibrate in the eardrum.
Sound travels through air, and putting your ear to a wall will not allow the person to hear the noise on the other side better. In such a case, the noise will be better heard when it travels through the air.
In conclusion, sound needs a medium such as air to travel.
Learn more about sound on:
https://brainly.com/question/9349349
Can someone help me with this
Answers
Answer:
2 is the answer
Please show me how you do and please involve the units

Answers
The question requires us to calculate the amount of energy absorbed by the reaction, considering the molar enthalpy for oxygen gas (O2) and that 80.6 g of oxygen were reacting.
The following information was provided by the question:
Balanced chemical reaction:
\(N_{2(g)}+2O_{2(g)}\to N_2O_{4(g)}\)Molar enthalpy of reaction for O2: +499.0 kJ/mol
Mass of O2 reacting: 80.6 g
To solve this problem, we need to calculate the amount of moles that corresponds to the mass of O2 given and then use this value and the molar enthalpy provided to calculate how much heat would be absorbed by the reaction.
First, we need the molar mass of O2. Knowing that the atomic mass of O is 15.99 u:
molar mass (O2) = (2 * 15.99) = 31.98 g/mol
Now, we use the molar mass to calculate the number of moles in 80.6 g of O2:
31.98 g O2 ------------------------ 1 mol O2
80.6 g O2 ------------------------- x
Solving for x, we'll have:
\(x=\frac{(80.6\text{ g O}_2)\times(1\text{ mol O}_2)}{(31.98\text{ g O}_2)}=2.52\text{ mol of O}_2\)There are 2.52 moles of O2 reacting and next we need to calculate the amount of heat absorbed considering this.
The molar enthalpy of O2 tells us how much heat is abosrbed when 1 mol of O2 reacts. Thus, we can use it to calculate the amount of heat absorbed when 2.52 moles of O2 react:
1 mol O2 ----------------------- 499.0 kJ
2.52 mol O2 ----------------- y
Solving for y, we'll have:
\(y=\frac{(2.52\text{ mol O}_2)\times(499.0\text{ kJ)}}{(1\text{ mol O}_2)}=1257.5\text{ kJ}\)Therefore, 1257.5 kJ of energy are absorbed when 80.6 g of O2 are reacting.
We can fill in the boxes to answer the question as it follows:
Answer: 1257.5
Units: kJ
Calculate the emf of the following concentration cell: Au(s) | Au3+ (0.10 M) || Au3+ (0.50 M) | Au(s)
Answers
The emf of the cell as written is calculated to be 0.014 V.
Using the Nernst equation;
Ecell = E°cell - 0.0592/n log Q
We must note that E°cell = 0 because the anode and cathode are composed of the same type of metal.
Now;
Substituting values, we have;
Ecell = 0 - 0.0592/3 log (0.50 M)/(0.10 M)
Ecell = 0.014 V
Learn more: https://brainly.com/question/15394851
if the volume of a cylinder is 84,300 cubic centimeters then how many millimeters is this equal to
Answers
Answer:
84,300,000
Explanation:
Just multiply it by 1,000. 1cm = 10mm, and a cube is the length to a sort of third degree, so you take the 10 to its third degree as well and multiply it by 1,000, instead of 10 like you would to find how many millimeters were in an amount of centimeters. If that makes sense.
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
What is the ionic
charge for this
element?
Answers
Answer:
To find the ionic charge of an element you'll need to consult your Periodic Table. On the Periodic Table metals (found on the left of the table) will be positive. Non-metals (found on the right) will be negative.Explanation:
mark as me brainliest24. The Henry’s law constant for O2 is 1.3 × 10−3 M/atm at 25 °C. Assuming ideal solution behavior, what mass of oxygen, in grams, would be dissolved in a 40. L aquarium at 25 °C, assuming an atmospheric pressure of 1.00 atm, and that the partial pressure of O2 is 0.21 atm?
Please enter your answer with two sig figs, no units, no scientific notation.
Explain please <3
Answers
The aquarium has 0.35 g of dissolved oxygen in it.
What is mass?The quantity of matter in an object is expressed in terms of mass. It is a scalar number, and units like grams, kilograms, and pounds are used to measure it. Weight, the force of gravity acting on an object, is not the same as mass. An object's mass is a fundamental characteristic that exists regardless of where it is or the gravitational environment it is in.
How do you determine it?Henry's law, which connects the concentration of a gas in a solution to its partial pressure, can be used to determine the mass of oxygen (O2) that would be dissolved in a 40 L aquarium:
C = kH x P
where P is the partial pressure of the gas, kH is the Henry's law constant, and C is the concentration of the gas in the solution.
To solve the concentration by rearranging the equation, we obtain:
C = (kH x P)
Using the above values for kH and P, we obtain:
C = (1.3 10^-3 M/atm) x (0.21 atm) = 2.73 10^-4 M.
This indicates that the aquarium's dissolved oxygen concentration is 2.73 x 10^-4 M.
We can use the following equation to determine the mass of O2 dissolved in the aquarium:
Mass = concentration x volume x molar mass.
where O2 has a molar mass of 32 g/mol.
Inputting the values provided yields:
mass = (2.73 x 10^-4 M) x 40 L x 32 g/mol = 0.35 g
As a result, the aquarium has 0.35 g of dissolved oxygen in it.
To know more about mass, visit:
brainly.com/question/15959704
#SPJ1
Determine the Density of the following objects mass= 15g volume = 2cm3
Answers
Answer:
7.5 g/cm³
Explanation:
density= mass / volume
density= 15 / 2= 7.5 g/cm³
hope it helps! please mark me brainliest
thank you! have a good day ahead
if you follow me, I will follow u back
it is a way of thinking about the world, and is made up of a person's views and belief
Answers
Incomplete question. I inferred you want the term that fits this definition.
Answer:
A Worldview
Explanation:
Having a Worldview refers to an individual's perception or the way one thinks about the world, influenced by their attitudes and values.
This view of the world is made up of a person's views and beliefs which form their expectations about the world in which they live.
In class we derived the Gibbs energy of mixing for a binary mixture of perfect gases. We also discussed that the same result is obtained for liquids when the resulting solution is ideal. For real solutions we introduced activities and activity coefficients. Derive the molar Gibbs energy of mixing and the molar excess Gibbs energy of mixing in terms of activity coefficients.
Answers
Answer:
Attached below
Explanation:
Free energy of mixing = ΔGmix = Gf - Gi
attached below is the required derivation of the
a) Molar Gibbs energy of mixing
ΔGmix = Gf - Gi
hence : ΔGmix = ∩RT ( X1 In X1 + X2 In X2 + X3 In X3 + ------- )
b) molar excess Gibbs energy of mixing
Ni = chemical potential of gas
fi = Fugacity
N°i = Chemical potential of gas when Fugacity = 1
ΔG = RT In ( a2 / a1 )

Look at the attachment below.

Answers
Sally is wrong because copper is less electropositive than hydrogen, thus, can not displace hydrogen from dilute acids.
The reactions to prepare copper (ii) chloride are:
the chlorination of copper sulfide at a high temperature
reaction of copper (ii) oxide with dilute hydrochloric acid
The equations of the given reactions are as follows:CuS + Cl₂ ---> CuCl₂ + SCuO + 2HCl ----> CuCl₂ + H₂O
What are reactive metals?Reactive metals are metals that readily give up their electrons to form positive ions.
Reactive metals displace hydrogen from dilute acids. They are found in group 1A and 2A of the periodic table. Copper is not a reactive metal and will not displace hydrogen from acids.
Learn more about reactive metals at: https://brainly.com/question/20273277
#SPJ1
Sally is wrong because copper chloride is not made from the reaction of copper and dilute hydrochloric acid.
2. Copper (ii) chloride can be prepared as follows:
reacting copper (ii) oxide with dilute hydrochloric acidsingle replacement reaction of copper sulfide and chlorine gas at a high temperature3. the equations of the reaction are:
CuO + 2HCl ----> CuCl₂ + H₂OCuS + Cl₂ ---> CuCl₂ + SWhat are single replacement reactions?Single replacement reactions are reactions in which a more reactive atom replaces another atom in a compound.
An example of a single replacement reaction is the reaction of chlorine gas with copper sulfide at high temperatures to form copper chloride.
Learn more about single replacement reaction at: https://brainly.com/question/20216315
#SPJ1
Explain how a rainbow is produced
Answers
A rainbow is produced through a proces that includes refraction, reflection, and dispersion of sunlight.
What more should you know about the production of rainbows?A rainbow is formed when sulinght is refracted and reflected by rain drops in the atmospher.
The sunlight is split into its component colors, which is why rainbows appear as having an array of colors. This is due to each color being bent by a different amount during refraction.
The colors of a rainbow are always in the same order, with red on the outside and violet on the inside.
Find more exercises on rainbows;
https://brainly.com/question/7965811
#SPJ1
The picture shows the cubic unit cell of CaF2. How many Ca2+ ions and F- ions are present in the cubic unit cell?
Answers
The total number of Ca2+ ions and F- ions that are present in the cubic unit cell will be 4 and 8 respectively.
How to calculate the unit cell?From the complete information, the number of Ca2+ at corners will be (8 × 1/8 = 1) and those a the center will be (6 × 1/2 = 3). Therefore, the total number of Ca2+ will be:
= 1 + 3 = 4
The total number of F- ions will be:
= 4 × 2 = 8
Learn more about unit cell on:
https://brainly.com/question/15070277
Consider the structure of chloride ion. Draw the conjugate acid for chloride ion. Remember to include charges and non-bonding electrons where necessary. Select Draw Rings More Erase H CI C17
Answers
The chloride ion (Cl-) has a single negative charge and a full octet of electrons in its outermost shell. It has a tetrahedral shape, with three equatorial lone pairs and one axial bond to a hydrogen or other positively charged ion.
The conjugate acid of chloride ion is HCl (hydrogen chloride), which is formed when the chloride ion accepts a proton (H+) from an acid. The resulting molecule has a positive charge and a linear shape, with a single bond between the hydrogen and chlorine atoms.
The conjugate acid of chloride ion, HCl, is a strong acid that readily donates a proton (H+) to a base to form the chloride ion. In water, HCl dissociates completely to form H+ and Cl- ions. The hydrogen ion (H+) has a positive charge and no electrons, while the chloride ion (Cl-) retains its original tetrahedral shape and three lone pairs of electrons.
Learn more about electrons here :
https://brainly.com/question/1255220
#SPJ4
I need help with this

Answers
As a result, the ideal gas law is applied, and the pressure of the gas in the container is 1.44 atm.
How does Charles Law compute pressure?The Kelvin temperature and hence the volume are going to be in direct proportion when the pressure on a sample of a dry gas is held constant, according to the definition of the Charles Law Formula. PV = k is the law's equation, and k might be a constant.
This issue can be resolved by applying the ideal gas law:
PV = nR
T = -52 °C + 273.15 = 221.15 K
n = 0.642 mol
V = 8.6 L
T = 221.15 K
\(R = 0.0821 L·atm/mol·K (gas constant for ideal gases)\)
PV = nRT
P = nRT/V
P = (0.642 mol)(0.0821 L·atm/mol·K)(221.15 K)/(8.6 L)
P = 1.44 atm
To know more about ideal gas visit:-
https://brainly.com/question/28257995
#SPJ1
3. Given the names of the following compounds, determine their correct chemical formula
e. Pentanitrogen tribromide
Answers
Answer:
N5Br3
Explanation:
Uhm cell parts and functions
Answers
A cell is the structural and fundamental unit of life. The study of cells from its basic structure to the functions of every cell organelle is called Cell Biology. Robert Hooke was the first Biologist who discovered cells
two types of cell
1) Prokaryotes
2) Eukaryotes
Characteristics of Cells
1) Cells provide structure and support to the body of an organism.
2) The cell interior is organised into different individual organelles surrounded by a separate membrane.
3) The nucleus (major organelle) holds genetic information necessary for reproduction and cell growth
\(hope \: its \: helpful \: to \: you \: please \: mark \: me \: a \: brainliest\)

A cell is defined as the fundamental, structural and functional unit of all life.
have a great day
God bless you

please help!!
62.4 mL of an H2SO3 solution
were titrated with 65.25 mL of a
0.235 M KOH solution to reach the
equivalence point. What is the
molarity of the H2SO3 solution?

Answers
The concentration of H₂SO₃ solution is equal to 0.123 M.
What is a neutralization reaction?A neutralization reaction is described as a reaction in which an acid and base react to form salt and water. When a strong acid will react with a base then the salt which is formed can be neither acidic nor basic.
When H₂SO₃ (a strong acid) reacts with KOH, the resulting salt is K₂SO₃ and water.
H₂SO₃ + 2KOH → K₂SO₃ + 2H₂O
Given, the concentration of KOH = 0.235 M
The volume of the KOH = 65.25 ml = 0.06525 L
The number of moles of KOH, n = M × V = 0.235 × 0.06525 = 0.0153 M
The volume of the H₂SO₃ = 62.4 ml = 0.0624 L
The number of moles of H₂SO₃, n = 0.0153/2 = 0.00767 mol
The concentration of H₂SO₃ =0.00767/0.0624 = 0.123 M
Therefore, the molarity of H₂SO₃ is 0.123 M.
Learn more about neutralization reaction, here:
brainly.com/question/20038776
#SPJ1
• How does the name of the salt tell us that:
a) there is just one other element combined with the metal?
b) there is oxygen present in the salt?
Answers
The name of the salt tells us that:
a) there is just one other element combined with the metal by looking at the suffix of the salt's name.
b) the presence of oxygen in a salt can be indicated by the name of the salt.
a) The name of a salt can tell us that there is just one other element combined with the metal by looking at the suffix of the salt's name. If the salt name ends in "-ide," it indicates that the salt is composed of a metal and a single non-metal element.
For example, sodium chloride (NaCl) and potassium bromide (KBr) are salts where the metal (sodium and potassium) is combined with a single non-metal element (chlorine and bromine, respectively). The "-ide" suffix suggests the presence of only one other element in the salt.
b) The presence of oxygen in a salt can be indicated by the name of the salt. If the salt name includes the element oxygen, it suggests that oxygen is present in the salt compound.
For example, sodium carbonate (Na₂CO₃) and calcium sulfate (CaSO₄) contain the element oxygen in their chemical formulas. The presence of oxygen in the salt is implied by the name and the combination of elements in the compound.
Therefore, the name of salt tells us that there is just one other element combined with the metal and there is oxygen present in the salt
Learn more about salt here:
https://brainly.com/question/31814919
#SPJ 1