Alexandra is preforming this combustion reaction i lab: C3H8 + 5O2 + 3CO + 4h20. If she carefully measures out 220 g of C3H8 and discovers she has produced 660 g of carbon dioxide and 360 g of water, how many grams of oxygen must have been required to react with the C3H8?
Answers
Answer:
800 g of O2.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
C3H8 + 5O2 —> 3CO2 + 4H2O
Next, we shall determine the masses of C3H8 and O2 that reacted from the balanced equation. This is illustrated below:
Molar mass of C3H8 = (12×3) + (8×1)
= 36 + 8
= 44 g/mol
Mass of C3H8 from the balanced equation = 1 × 44 = 44 g
Molar mass of O2 = 16 × 2 = 32 g/mol
Mass of O2 from the balanced equation = 5 × 32 = 160 g
From the balanced equation above,
44 g of C3H8 reacted with 160 g of O2.
Finally, we shall determine the mass of O2 needed to react with 220 g of C3H8. This can be obtained as follow:
From the balanced equation above,
44 g of C3H8 reacted with 160 g of O2.
Therefore, 220 g of C3H8 will react with = (220 × 160)/44 = 800 g of O2.
Thus, 800 g of O2 is needed for the reaction.
Related Questions
What is the most common ionic form of fluorine?
Answers
Answer: It will usually form the anion F- since it is extremely electronegative and a strong oxidizing agent. Fluorine is a Lewis acid in weak acid, which means that it accepts electrons when reacting. Fluorine has many isotopes, but the only stable one found in nature is F-19.
Explanation:
The most common ionic form of fluorine is the fluoride ion (F-). Fluorine, as an element, is highly electronegative, meaning it has a strong tendency to attract electrons towards itself.
In the process of forming an ion, fluorine gains one electron to achieve a stable electron configuration, resulting in the fluoride ion.
For example, when fluorine reacts with sodium (Na), fluorine gains one electron from sodium to form the fluoride ion (F-) while sodium loses one electron to become the sodium cation (Na+). This forms an ionic bond between the two ions, resulting in the compound sodium fluoride (NaF).
The fluoride ion is also commonly found in compounds such as calcium fluoride and aluminum fluoride . In these compounds, fluorine forms ionic bonds with other elements, resulting in the formation of stable compounds.
Overall, the fluoride ion is the most common ionic form of fluorine due to its high electronegativity and its ability to form stable compounds with other elements.
Learn more about fluorine,here:
https://brainly.com/question/1940697
#SPJ6
Can two (or more) types of matter occupy the same space at the same time?
Answers
The general properties of matter result from its relationship with mass and space. ... Because it occupies space, all matter has volume and impenetrability, since two objects cannot occupy the same space simultaneously.
based on the rcp2.6 scenario, what is the predicted atmospheric co2 concentration in 2100, and the global temperature change and sea level change from 2000 to 2100?
Answers
The expected CO2 concentration will be around 400 ppm in 2100.
What do you mean by RCP?
RCP's can be defined as the Representative concentration pathway which is usually a climate model to describe the different radiative forcing depending on the CO2 concentrations.
The above case studies states that:
The RCP 2.6 scenario is "rise and fall" scenario in which the radiative forcings will rise up to about 3.1W/m2 by 2050 and then decline to 2.6W/m2 by 2100. The CO2 concentrations are expected to be within 400 ppm in this model. Oceans will warm at a slower pace and most of the sea level rise is expected from melting of ice sheets.
Hence, The expected CO2 concentration will be around 400 ppm in 2100.
To know more about CO2 from the given link
https://brainly.com/question/16596799
#SPJ4
Calculate the volume of ethyl alcohol that evaporated (in mL). 1 L = 1,000 mL and 1 mole of ANY gas at STP = 22.4 L.
Answers
To calculate the volume of ethyl alcohol that evaporated, we need to know the volume of ethyl alcohol that was present initially, the volume of ethyl alcohol after the evaporation, and the molar mass of ethyl alcohol.
Let's assume that the initial volume of ethyl alcohol is V_0 mL and the final volume is V_f mL. The volume of ethyl alcohol that evaporated is then V_e = V_0 - V_f mL.
We can use the ideal gas law to relate the volume of a gas to the number of moles of the gas present. The ideal gas law is given by:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
Since the volume of a gas is directly proportional to the number of moles of the gas, we can write:
V/n = k
where k is a proportionality constant.
If we know the value of k for a given gas at a particular temperature and pressure, we can use this relationship to calculate the number of moles of the gas present.
In this case, we are told that 1 mole of any gas at STP (standard temperature and pressure) occupies a volume of 22.4 L, which is equivalent to 22,400 mL. Therefore, the value of k for any gas at STP is 22,400 mL/mol.
We can use this value of k to calculate the number of moles of ethyl alcohol that evaporated:
n = V_e/(22,400 mL/mol)
Substituting in the known values for V_e and k, we can calculate the number of moles of ethyl alcohol that evaporated.
For example, if the initial volume of ethyl alcohol was 1,000 mL and the final volume was 900 mL, the volume of ethyl alcohol that evaporated would be V_e = 1,000 mL - 900 mL = 100 mL. The number of moles of ethyl alcohol that evaporated would be:
n = 100 mL/(22,400 mL/mol) = 0.0044 moles
I hope this helps! Let me know if you have any questions.
2.28 Global Warming’s Negative Effects Worksheet
Watch this Video and to answer the following questions.
What type of human activity increases the amount of CO2 in the atmosphere?
Explain how climate change affects our oceans
Explain how climate change affects our weather
Explain how climate change affects our food
Explain how climate change affects our health
What can we do to prevent the negative effects of climate change?
PLEASE ANSWER ASAP GIVE BRAINLIEST
Answers
Climate change causes the oceans to absorb more heat, resulting in an increase in sea surface temperatures and rising sea levels. This change in ocean temperatures and currents brought about by climate change can lead to alterations in climate around the world.
Climate changes affects crop growth, meat production, fisheries, and other fundamental aspects of our food supply. This is because if our climate is constantly changing our Earth will no longer be able to sustain the adequate necessities of food production.
Climate change cause health issues. Change in climate can cause existing health problems to intensify and new health issues to emerge. Climate change can influence air pollution, allergies, disease carried by vectors, food and waterborne diseases, food security, mental health and stress-related disorders, floods, temperature extremes, and wildfires. All of these factors can have negative effects on ones health.
To reduce the negative effects of climate change we can speak up about climate change, use renewable energy, weatherize, invest in energy-efficient appliances, reduce water waste, make less food waste, etc.
help please
Assuming that the trends continue, which of the following compounds do you predict will have the GREATEST solubility at 120°C?
A.
Ce2(SO4)3
B.
K2Cr2O7
C.
Pb(NO3)2
D.
NaCl

Answers
Answer:
K2Cr2O7
Explanation:
Solubility refers to the amount of substance that dissolves in a given mass or volume of solvent. There are several units of solubility applicable in different areas.
Solubility is dependent on temperature. The solubility curve is a graphical representation of the dependence of solubility on temperature for different chemical species.
If we study the solubility curve closely, we will see that K2Cr2O7 has the highest solubility at 100°C. This means that if the trends continue, this substance will also have the highest solubility at 120°C.
Answer: c
Explanation:
N204(0) + 2NO2(g)
Colorless Brown
Keq = 6.16 x 103
What is the predicted direction of change?
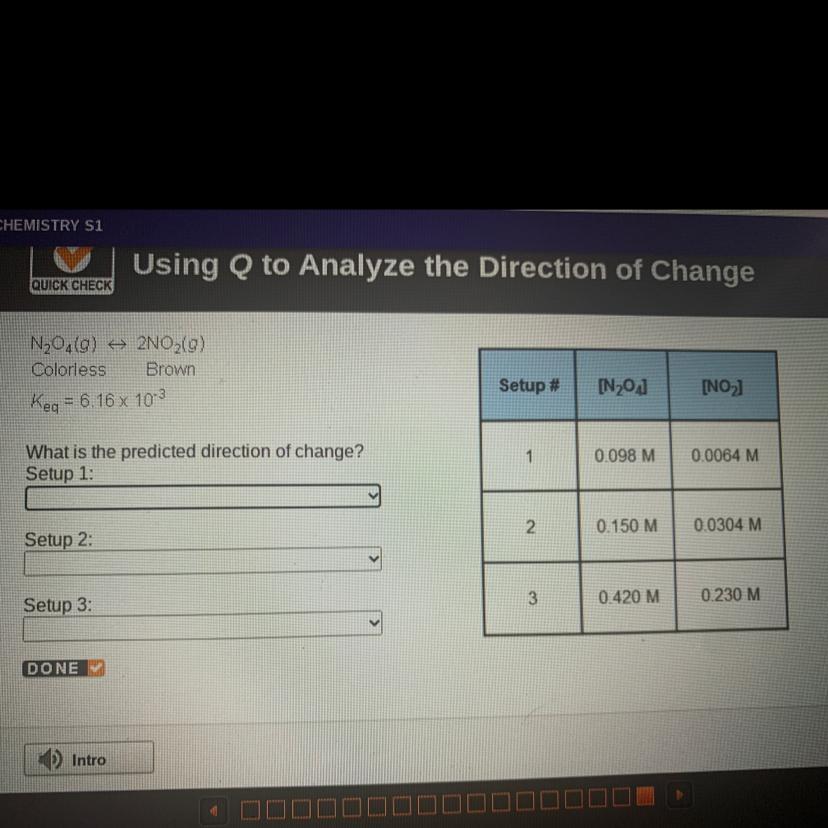
Answers
setup 1 : to the right
setup 2 : equilibrium
setup 3 : to the left
Further explanationThe reaction quotient (Q) : determine a reaction has reached equilibrium
For reaction :
aA+bB⇔cC+dD
\(\tt Q=\dfrac{C]^c[D]^d}{[A]^a[B]^b}\)
Comparing Q with K( the equilibrium constant) :
K is the product of ions in an equilibrium saturated state
Q is the product of the ion ions from the reacting substance
Q <K = solution has not occurred precipitation, the ratio of the products to reactants is less than the ratio at equilibrium. The reaction moved to the right (products)
Q = Ksp = saturated solution, exactly the precipitate will occur, the system at equilibrium
Q> K = sediment solution, the ratio of the products to reactants is greater than the ratio at equilibrium. The reaction moved to the left (reactants)
Keq = 6.16 x 10⁻³
Q for reaction N₂O₄(0) ⇒ 2NO₂(g)
\(\tt Q=\dfrac{[NO_2]^2}{[N_2O_4]}\)
Setup 1 :
\(\tt Q=\dfrac{0.0064^2}{0.098}=0.000418=4.18\times 10^{-4}\)
Q<K⇒The reaction moved to the right (products)
Setup 2 :
\(\tt Q=\dfrac{0.0304^2}{0.15}=0.00616=6.16\times 10^{-3}\)
Q=K⇒the system at equilibrium
Setup 3 :
\(\tt Q=\dfrac{0.230^2}{0.420}=0.126\)
Q>K⇒The reaction moved to the left (reactants)
Answer:
The system will shift toward the products
The system is at equilibrium
The system will shift toward the reactants
Explanation:
This is correct on edg... Good Luck!!!!
Which one is a compound

Answers
Answer:
CO
Explanation:
the rest are elements. CO is made up of one carbon atom and one oxygen atom
Help please if link will report

Answers
Balance the equation choose the coefficient for blank 3 (in front of KBr)___ KOH + ___ HBr --> ___ KBr + ___ H2O
Answers
Balancing the equation :
KOH(aq) + HBr(aq) --> KBr(aq) + H2O(l)
This is the balanced chemical reaction because it follows the following ionic reaction:
H ^(aq)+ + Br^(aq)- + K^+(aq) + OH ^- (aq) → K^+(aq) + Br^-(aq) + H2O (l)
the molecular formula for glucose is . what would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions?
Answers
c.) C60H102O51 be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions
Large molecules known as macromolecules are made up of several atoms that are joined together covalently. Since polymers include tens of thousands of atoms, they fall into the macromolecular category. Biopolymers, which comprise proteins, carbohydrates, and nucleic acids, are the most prevalent type of polymers. Consequently, we might say that the phrase that best represents polymers is Macromolecular entities are polymers. A large percentage of synthetic polymers are not biodegradable (unlike natural fibres such as cotton). According to their intended purpose, synthetic polymers can be divided into plastics, elastomers, and synthetic fibres. Synthetic polymers have the benefits of being tough to break, lightweight, and long-lasting. In conclusion, the lightweight nature of synthetic polymer makes them advantageous for use.
The molecular formula for glucose is . what would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions?
a.) C60H120O60
b.) C6H12O6
c.) C60H102O51
d.) C60H100O50
e.) C60H111O51
Learn more about polymer here:
https://brainly.com/question/17555341
#SPJ4
Find out about carbon explain how it’s properties make it suitable for uses
Answers
A sample of aluminum has mass 26.98 g and has a density of
2.70 g/cm3. Calculate volume to the correct number of significant
figures.
Answers
Answer:
9.99L
Explanation:
you can see the answer at the pic

Answer:
The volume of aluminum is 9.99 cm³.
Explanation:
Given data:
Mass of aluminum = 26.98 g
Density of aluminum = 2.70 g/cm³
Volume of aluminum = ?
Solution:
Formula:
d = m/v
now we will put the values in formula.
2.70 g/cm³ = 26.98 g / v
v = 26.98 g / 2.70 g/cm³
v = 9.99 cm³
Thus, the volume of aluminum is 9.99 cm³.
SHOW WORK
Calculate the morality
10.0 g of sodium chloride is dissolved in 2.0 L of solution.
Answers
How many moles are there in 105.69 grams of FeCI2
Answers
Answer:
0.8338395752301793
Explanation:
I used an online converter (grams to moles for FeCl2)
The elementary gas phase reaction 2A + B --> 2C (irreversible
reaction) Is carried out isothermally in a PFR with no pressure
drop. The feed is equal molar in A and B and the entering
concentration
Answers
The given reaction, 2A + B -> 2C, is an elementary gas-phase reaction that is irreversible. It is being carried out isothermally in a plug flow reactor (PFR) with no pressure drop.
In a PFR, the reaction takes place as the reactants flow through the reactor continuously without any back-mixing. This allows for a steady-state concentration profile along the reactor length.
Since the reaction is irreversible, the conversion of A and B to C will occur as the reactants flow through the reactor. As the reaction progresses, the concentrations of A and B will decrease, while the concentration of C will increase.
Because there is no pressure drop in the reactor, the reaction is not influenced by changes in pressure. The reaction rate will depend solely on the reactant concentrations and temperature.
To determine the behavior of the reaction in terms of conversion and concentration profiles along the reactor, additional information such as the reaction rate constant and reactor volume would be required.
Overall, the given information states that in an isothermal PFR with no pressure drop, the equal molar feed of A and B will lead to the formation of an equal amount of C as the reaction progresses. The specific details of the conversion and concentration profiles would depend on additional parameters and can be determined with the appropriate rate equation and reactor design.
To learn more about isothermally
https://brainly.com/question/28199016
#SPJ11
balance the following equation.
C + H2 + 02 → C2H60
Answers
Answer:
4C + 6H2 + O2>>2C2H6OExplanation:
there must be equal number of carbons, hydrogen and oxygen between products and reactants
if 6 moles of a a compound produce 84 J of energy, what is the h reaction in j/mol
Answers
The enthalpy of the reaction is 14 J/mol.
The enthalpy of a reaction (ΔH) is the amount of energy transferred between a system and its surroundings during a chemical reaction at constant pressure, measured in joules per mole (J/mol). This value is important because it can tell us whether a reaction is exothermic or endothermic, as well as give us information about the strength of chemical bonds within the reactants and products.To calculate the enthalpy of a reaction, we need to know the amount of energy released or absorbed (Q) and the number of moles of the compound involved in the reaction (n). We can use the equation:
ΔH = Q/n
Given that 6 moles of a compound produce 84 J of energy, we can calculate the enthalpy of the reaction as follows:
ΔH = Q/n
ΔH = 84 J / 6 mol
ΔH = 14 J/mol
This means that for every mole of the compound involved in the reaction, 14 J of energy is transferred between the system and the surroundings. Since the value is positive, we can conclude that the reaction is endothermic, meaning that it requires an input of energy to occur.It is worth noting that the enthalpy of a reaction can depend on a number of factors, such as temperature, pressure, and the specific conditions under which the reaction occurs. As such, it is important to take these factors into account when calculating or predicting enthalpy values.
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
If an atom has a mass number of 52 and has 28 neutrons, what is the atomic number?
Answers
Answer:
24
Explanation:
52-28=24
Use the drop-down menus to select the sentences that show precise use of pronouns.
1. Fred visited Nick after his graduation.
2. After Nick's graduation, Fred visited him.
1. Marie told Sarah that she makes great spaghetti.
2. Marie told Sarah, "You make great spaghetti."
1. John broke the fence with his bike.
2. John's bike hit a fence and it broke.
Answers
Sentence 1
Sentence2
Answers
Answer:
sentence 2, sentence 2, sentence 1
Explanation:
it was right for me on edge 2020
Answer:
1. Fred visited Nick after his graduation.
2. After Nick's graduation, Fred visited him.
1. Marie told Sarah that she makes great spaghetti.
2. Marie told Sarah, "You make great spaghetti."
1. John broke the fence with his bike.
2. John's bike hit a fence and it broke.
which of the four substances listed will dissolve in water and why?
Answers
Answer:
Sugar, sodium chloride, and hydrophilic proteins are all substances that dissolve in water. Oils, fats, and certain organic solvents do not dissolve in water because they are hydrophobic.
And, water is called the "universal solvent" because it dissolves more substances than any other liquid. ... Water molecules have a polar arrangement of the oxygen and hydrogen atoms—one side (hydrogen) has a positive electrical charge and the other side (oxygen) had a negative charge.
I don't see any options so there i hope it helps .
12. What is the atomic number of the atom that forms an anion with 36 electrons and a Explain(words); write out formula, then solve for missing variable charge of -1?
Answers
The missing variable of (⁻¹) is bromine.
Let the missing variable be X.
No. of electrons in the monoatomic anion (X⁻¹ )= 36
No. of electrons in the monoatomic atom (X) = 35
Atomic No. = No. of protons = No. of electrons in neutral atom = 35
Mass No. = No. of protons + No. of neutrons
= 35 + 45 = 80
Hence, the element must be Br as its mass no is 80 and atomic no is 35.
And the ion is bromide ion or Br⁻¹ ion. It is an anion which gains an electron to achieve octet.
X → Br and X⁻¹ → Br⁻¹
Learn more about bromine from the link given below.
https://brainly.com/question/4839867
#SPJ1
Round all answers on this assignment to exactly three decimal places (including any 0's on end).
Pick a random real number between 16.3 and 24.3:
The probability that the number will be exactly 18 is 0
The probability that the number will be between 18 and 20, inclusive, is
The probability that the number will be between 18 and 20, exclusive, is
The probability that the number will be less than 18 or greater than 20 is
The probability that the number will be less than 20 or greater than 18 is 1
Answers
Explanation:
Since we are asked to round all answers to three decimal places, we will use the rounded values in our calculations.
To find the probability for each case, we need to calculate the length of the interval divided by the length of the total range.
1. The probability that the number will be exactly 18 is 0. Since the number is continuous, the probability of selecting a single value is zero.
2. The probability that the number will be between 18 and 20, inclusive, is (20 - 18.3) / (24.3 - 16.3) = 1.7 / 8 = 0.212.
3. The probability that the number will be between 18 and 20, exclusive, is (20 - 18) / (24.3 - 16.3) = 2 / 8 = 0.250.
4. The probability that the number will be less than 18 or greater than 20 is ((16.3 - 18) + (24.3 - 20)) / (24.3 - 16.3) = (-1.7 + 4.3) / 8 = 2.6 / 8 = 0.325.
5. The probability that the number will be less than 20 or greater than 18 is ((16.3 - 18.3) + (24.3 - 20)) / (24.3 - 16.3) = (-2 + 4.3) / 8 = 2.3 / 8 = 0.288.
Therefore, the probabilities are as follows:
- The probability that the number will be exactly 18 is 0.
- The probability that the number will be between 18 and 20, inclusive, is 0.212.
- The probability that the number will be between 18 and 20, exclusive, is 0.250.
- The probability that the number will be less than 18 or greater than 20 is 0.325.
- The probability that the number will be less than 20 or greater than 18 is 0.288.
what is the main purpose of creating the serial dilutions of the stock solution?
Answers
The main purpose of creating serial dilutions of a stock solution is to obtain a series of solutions with different concentrations that are suitable for various analytical applications.
Serial dilutions involve the stepwise dilution of a concentrated stock solution with a solvent, such as distilled water or buffer solution. The result is a series of solutions with decreasing concentrations, which are typically prepared in a logarithmic fashion, such as 1:10 or 1:100 dilution factors.
Serial dilutions are useful in a variety of applications, such as in microbiology for counting bacteria or in biochemistry for enzyme kinetics. In microbiology, serial dilutions are used to dilute bacterial or fungal cultures to obtain a known concentration of cells that can be counted using a microscope or plate count method.
To know more about stock solution here
https://brainly.com/question/17018950
#SPJ4
if this atom has one valence electron, what kind of bond is it most likely to form with another atom? how many valence electrons might an atom that it bonds to have?
Answers
If an atom has one valence electron, it is most likely to form an ionic bond with another atom. The atom that bonds to the atom with one valence electron might have either seven or eight valence electrons.
Ionic bonds occur when one atom transfers its valence electron to another atom. In this case, the atom with one valence electron will likely donate it to the other atom.
The atom that bonds to the atom with one valence electron might have either seven or eight valence electrons. This is because atoms tend to gain or lose electrons in order to achieve a stable electron configuration.
If the atom with one valence electron bonds with an atom that has seven valence electrons, the atom with one valence electron will donate its electron to the other atom, resulting in both atoms achieving a stable configuration.
If the atom with one valence electron bonds with an atom that has eight valence electrons, the atom with one valence electron will transfer its electron to the other atom, resulting in the atom with eight valence electrons achieving a stable configuration.
In both cases, the formation of an ionic bond between these atoms allows them to achieve a stable electron configuration.
Learn more about valence electron -
brainly.com/question/371590
#SPJ11
5. Predict You are floating motionless on a
rubber raft in the middle of a pool. A friend
forms a wave by slapping the water every
second. Will the wave carry you to the edge
of the pool? Explain your answer.
Answers
When the pool's center is occupied by the rubber raft. Think of it as the raft's starting position.
Once it reaches the rubber raft, the raft will start to rise when the friend creates a wave by slapping the water.
The raft will really gain energy from the wave, moving upward.
The raft will return to its original place once the wave passes.
The raft will return to its original place where it was before the wave hits it because it is indicated in the question that it is immobile.
As a result, the wave won't take it to the pool's edge.
Because you are only lightly lifted by the waves or because Lee cannot be pushed to the other side of the pool, the wave won't drive the person on the raft over the edge of the pool.
To know more about waves, click on the link below:
https://brainly.com/question/11350613
#SPJ9
Which molecule has the shortest carbon-oxygen bond length?
A. CH3COOH
B. CH3CH2OH
C. CO₂
D. CO
Answers
On which slope would you expect to find the least precipitation if winds blow against a mountain from the south?
west
east
south
north
Answers
Answer: North
Explanation:
A bauxite mining company has got government permission to acquire agricultural land in a location to start surface mining activities. Which of these is the best plan to solve the problem of permanent dislocation of farmers due to mining in the location?
Answers
Answer:
make reclamation compulsory after bauxite has been removed
Explanation:
Available options:
choose other infertile and uninhabited locations for bauxite mining
allow agriculture and mining to take place simultaneously in the location
ask farmers in the location to help in mining bauxite
make reclamation compulsory after bauxite has been removed
The correct option and the best plan to solve the problem of permanent dislocation of farmers would be to make reclamation compulsory after the bauxite has been removed.
Reclamation would involve restoring the agricultural land back to its original status prior to the commencement of bauxite mining. By doing this, the farmers can return to their farmland after being temporarily displaced due to the mining activities.
answer is b
please mark brainliest
What do theories help scientists do? (1 point)
A. prove hypotheses to be correct
B. explain large collections of data
C. determine if a claim is false
D. propose new ideas on the subject
Answers
Answer:
A. prove hypotheses to be correct is the correct answer I think
Explanation:
A scientific theory has been defined as a thorough explanation of the facts and includes the hypothesis and laws. The scientific theory allows scientists to prove the hypothesis to be correct. Thus, option A is correct.
What are scientific theories?Scientific theories have been the summary of the universal and natural sciences that are well sustained and never proved. They are based on observable explanations and information that were tested through experimentation.
It uses experimental and research setup to prove the hypothesis to be credible by the conclusions and findings that are also supported by the scientific laws. They are reliable conclusions and are just not guesses.
Therefore, option A. scientific theories are used to prove the reliability of the hypothesis.
Learn more about scientific theories, here:
https://brainly.com/question/17050172
#SPJ2