Answers
The number of mole of carbon in 18.27 g of alanine is 0.615 mole
Determination of the mole of 18.27 g of alanineMolar mass of C₃H₇NO₂ = (3×12) + (7×1) + 14 + (2×16) = 89 g/mol Mass of C₃H₇NO₂ = 18.27 gMole of C₃H₇NO₂ =?Mole = mass / molar mass
Mole of C₃H₇NO₂ = 18.27 / 89
Mole of C₃H₇NO₂ = 0.205 mole
How to determine the number of mole carbon in 18.27 g (i.e 0.205 mole) of alanineAlanine => C₃H₇NO₂
From the formula of alanine,
1 mole of C₃H₇NO₂ contained 3 moles of C.
Therefore,
0.205 mole of C₃H₇NO₂ will contain = 0.205 × 3 = 0.615 mole of C
Thus, 0.615 mole of C is present in 18.27 g (i.e 0.205 mole) of alanine.
Learn more about mass composition:
https://brainly.com/question/13531044
Related Questions
An atom has 11 protons, 12 neutrons, and 10 electrons. What is the charge?
Answers
Answer:
The atom is Na, sodium with a net charge of +1 because it lost an electron. it usually has 11 electrons.
Explanation:
sodium has a 11 protons with an atomic mass of 23-11=12 neutrons.
To see the number of atoms of an element in a given molecule we need to multiply stoichiometry to the number that is written on the foot of the element that is stoichiometry. Therefore, the charge on atom is +1.
What is atom?Atom is the smallest particle of any element, molecule or compound. Atom can not be further divided. Atoms contains nucleus in its center and electron that revolve around the atom in fixed orbit.
In the nucleus, proton and neutron are present. Electron has -1 charge while proton has +1 charge. Neutron is neutral that is it has no charge. So overall the charge of nucleus is due to only proton, not by neutron.
Since the number of electrons is 10. The number of protons is 11. So, there is loss of one electron, so the charge on atom is +1.
Therefore, the charge on atom is +1.
To know more about atom, here:
https://brainly.com/question/13518322
#SPJ2
Adding 1.56 g of K2SO4 to 6.00 mL of water at 16.2ºC causes the temperature of the solution to drop by 7.70ºC.
How many grams of NaOH (ΔHsoln = –44.3 kJ/mol) would you need to add to raise the temperature back to 16.2ºC?
Answers
Answer:
You need to add 0.243g of NaOH to raise the temperature back to 16.2°C
Explanation:
Using the equation:
Q = C*m*ΔT
Where Q is heat
C is specific heat
m is mass
and ΔT is change in temperature
We can find the heat required to increase the temperature of the solution back to 16.2°C:
Assuming specific heat of the solution of water + K2SO4 = Specific heat of water:
C = 4.184J/g°C
m = 1.56g + 6.00g = 7.56g
ΔT = 16.2°C - 7.70°C = 8.50°C
Q = 4.184J/g°C * 7.56g * 8.50°C
Q = 268.86J = 0.269kJ of heat are required
As this heat is obtained from the dissolution of NaOH:
0.269kJ * (1mol NaOH / 44.3kJ) = 0.00607 moles of NaOH are required
In grams -Molar mass NaOH: 40g/mol-:
0.00607 moles NaOH * (40g / mol) =
You need to add 0.243g of NaOH to raise the temperature back to 16.2°C..................
are called energy giving nutrients
Answers
Answer:
macronutrients (carbohydrates, lipids, and proteins)
What trophic level has heterotrophs?
a only the second level
b all levels except the first
C only the first level
d only the last level
Answers
10)
How many total atoms (N + H) are in one mole of ammonia (NH3) molecules?
A)
6.0x10^23
B)
1.2x10^24
C)
1.8x10^24
9
D)
2.4x10^24
Answers
Answer:
D
Explanation:
Took the test
Answer:
D
Explanation:
Got it right on my test
Iron reacts with chlorine to form iron(III) chloride.
2Fe + 3Cl2 → 2FeCl3
What mass (in grams) of chlorine gas is needed to react with 251 grams of iron?
Select one:
a.
71 grams
b.
392 grams
c.
479 grams
d.
622 grams
Answers
The mass (in grams) of chlorine gas is needed to react with 251 grams of iron is 479 grams. Option C.
To determine the mass of chlorine gas needed to react with 251 grams of iron, we need to use the stoichiometry of the balanced chemical equation:
2Fe + 3Cl2 → 2FeCl3
From the balanced equation, we can see that 2 moles of iron (Fe) react with 3 moles of chlorine gas (Cl2) to produce 2 moles of iron(III) chloride (FeCl3).
To calculate the mass of chlorine gas, we can follow these steps:
Step 1: Convert the given mass of iron (Fe) to moles.
Using the molar mass of iron (Fe), which is approximately 55.85 g/mol, we can calculate the number of moles of iron:
moles of Fe = mass of Fe / molar mass of Fe
moles of Fe = 251 g / 55.85 g/mol
moles of Fe ≈ 4.5 mol (rounded to one decimal place)
Step 2: Use the mole ratio from the balanced equation to find the moles of chlorine gas (Cl2) needed.
From the balanced equation, we know that 2 moles of Fe react with 3 moles of Cl2. Therefore, the moles of Cl2 can be calculated as:
moles of Cl2 = (moles of Fe / 2) * 3
moles of Cl2 = (4.5 mol / 2) * 3
moles of Cl2 ≈ 6.75 mol (rounded to two decimal places)
Step 3: Convert the moles of chlorine gas to grams.
Using the molar mass of chlorine gas (Cl2), which is approximately 70.90 g/mol, we can calculate the mass of chlorine gas:
mass of Cl2 = moles of Cl2 * molar mass of Cl2
mass of Cl2 = 6.75 mol * 70.90 g/mol
mass of Cl2 ≈ 479 grams (rounded to the nearest whole number) Option C is correct.
For more such question on mass. visit :
https://brainly.com/question/19385703
#SPJ8
What is the purpose of the arrow in a chemical equation?
Answers
The arrow in a chemical equation represents the direction of the reaction. It indicates the conversion of reactants into products. The arrow points from the reactant side to the product side, symbolizing the flow of the reaction.
The purpose of the arrow is to visually represent the chemical transformation occurring in the reaction. It shows the relationship between the reactants and products and the direction in which the reaction proceeds. The arrow implies that the reactant molecules are being rearranged and transformed into new substances with different properties.
Chemical equations are used to describe the stoichiometry and balance of reactions. The arrow helps convey this information by illustrating the overall process taking place. It serves as a crucial element in understanding the reaction's composition, reaction conditions, and the substances involved.
Furthermore, the arrow also implies that the reaction can occur in both directions. In reversible reactions, the arrow can be represented as a double-headed arrow, indicating that the reaction can proceed in either direction depending on the conditions.
Know more about reversible reactions here:
https://brainly.com/question/21426719
#SPJ8
why do you have to pay for everthing it should be free for new user for like a week
Answers
What is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the Hg2 concentration is 7.36E-4 M and the Al3 concentration is 1.05 M
Answers
Answer:
Explanation:
Concentration of Hg⁺² = 7.36 x 10⁻⁴ M
Concentration of Al⁺³ = 1.05 M
2Al + 3Hg⁺² = 2Al⁺³ + 3Hg .
E = E₀ + RT / nF ln [ Al⁺³]² / [ Hg⁺² ]³
E₀ = reduction potential of Hg⁺² minus reduction potential of Al⁺³
= 0.92 V - ( - 1.66 V )
= 2.58 V
E = 2.58 + .059 /n log [ Al⁺³]² / [ Hg⁺² ]³
n = 6 , [Al⁺³] = 1.05 M ; [Hg⁺²] = 7.36 x 10⁻⁴ M
E = 2.58 + .059 /6 log [ 1.05]² / [ 7.36 x 10⁻⁴ ]³
= 2.58 + .059 /6 log 27.65 x 10⁸ .
= 2.58 + .059 /6 [8+ log 27.65 ].
= 2.58 + .059 /6 [8+ log 27.65 ].
= 2.58 + .09
= 2.67 V .
What volume of 0.300 M NaOH solution is required to neutralize 32.5c * m ^ 3 of a 0.180 M HCI solution?
Answers
According to molar concentration, 19.5 ml of 0.300 M NaOH solution is required to neutralize 32.5 cm³ of a 0.180 M HCI solution.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.
In the given problem, volume of NaOH is found out by the formula M₁V₁=M₂V₂ substitution in the given formula gives, V₁=0.180×32.5/0.3=19.5 ml.
Thus, 19.5 ml of 0.300 M NaOH solution is required to neutralize 32.5 cm³ of a 0.180 M HCI solution.
Learn more about molar concentration,here:
https://brainly.com/question/21841645
#SPJ1
If a sample of glucose (C6H12O6 molecular mass = 180.12 amu/molecule) weighs 4863 amu, how many molecules are in the sample?
Round and enter this answer to ones place. For example ... if the calculated answer was 1546.4, enter 1546
Answers
The number of molecules in a sample of glucose that weighs 4863 amu is 1.6253 × 10²¹ molecules.
How to calculate number of molecules?The number of molecules in a substance can be calculated by multiplying the number of moles in the substance by Avogadro's number as follows:
no of molecules = no of moles × Avogadro's number
According to this question, a sample of glucose (C6H12O6 molecular mass = 180.12 amu/molecule) weighs 4863 amu. The moles of this sample of glucose can be calculated as follows:
moles = 4863amu ÷ 180.12 amu/molecule
moles = 26.99 moles
No of molecules = 26.99 × 6.02 × 10²³
No of molecules = 1.6253 × 10²¹ molecules.
Learn more about no of molecules at: https://brainly.com/question/19481036
#SPJ1
Based on Robert's knowledge of physical properties of matter, describe how he can do this?
Answers
Matter of the substance is the mass and takes up space, volume . .The physical properties of matter is the that determine without changing chemical identity.
The properties of matter is of two types : the physical property and the chemical property . A physical properties of matter is the property that can be determined without changing their chemical identity of a substance. example : density , color, mass , volume , length, etc. the physical properties are of two types : intensive physical properties and the extensive physical properties.
The chemical properties of that that can be determine by the changing the chemical identity of substance. example : toxicity, heat, enthalpy, flammability.
Thus, Matter of the substance is the mass and takes up space, volume .The physical properties of matter is the that determine without changing chemical identity.
To learn more about physical properties here
https://brainly.com/question/19532631
#SPJ1
When population growth levels off in an ecosystem, it has reached its _________.
Answers
it represents the maximum population size a particular environment can support
The average speeds of gas molecules in cylinders A, B, C, and D are 0.03 m/s, 0.009 m/s, 0.1 m/s, and 1.5 m/s respectively.
Which cylinder contains gas that is closest to absolute zero?
C
B
A
D
Answers
The average speeds of gas molecules in cylinders A, B, C, and D are 0.03 m/s, 0.009 m/s, 0.1 m/s, and 1.5 m/s respectively. Cylinder A contains gas that is closest to absolute zero.
What is absolute zero temperature?Absolute zero, often known as 0 kelvin, is the lowest point on the thermodynamic temperature scale, when the enthalpy as well as entropy of a cooled ideal gas reach their lowest values.
According to international treaty, absolute zero is defined as 273.15 degrees Celsius, which really is equivalent to 459.67 degrees Fahrenheit. The average speeds of gas molecules in cylinders A, B, C, and D are 0.03 m/s, 0.009 m/s, 0.1 m/s, and 1.5 m/s respectively. Cylinder A contains gas that is closest to absolute zero.
Therefore, Cylinder A contains gas that is closest to absolute zero.
To know more about absolute zero temperature, here:
https://brainly.com/question/14327817
#SPJ1
What is the relationship between chromosomes and DNA?
Question 3 options:
Chromosomes are made of DNA
DNA is made of chromosomes
Chromosomes manufacture DNA
They are on a break..
Answers
Answer:
answer is
chromosomes are made of
What are the the basic cloud types?
Answers
Answer:
they are 10 but I'll be listing three
Explanation:
cirrus,cirrocumulus and cirrostratus
I hope this helps....good luck with the rest...again, I hope this helps
A solution of aluminum chloride has a pH of (4.5x10^0). What is the [H3O*(aq)], in mol/L?
Note: Your answer is assumed to be reduced to the highest power possible.
Answers
The concentration of H3O+ ions in the solution of aluminum chloride is \(3.16×10^-5\) mol/L.
Aluminum chloride is an acidic salt that contains a cation, Al3+, and an anion, Cl-. When aluminum chloride is dissolved in water, it dissociates into its constituent ions, and the Al3+ cations hydrolyze to produce H+ ions.
This reaction leads to the formation of an acidic solution. The pH of a solution of aluminum chloride is \(4.5×10^0\). We need to determine the concentration of H3O+ ions in this solution.
The concentration of H3O+ ions in a solution is given by the equation: pH = -log[H3O+] where pH is the negative logarithm of the concentration of H3O+ ions in the solution. The negative sign indicates that the pH is inversely proportional to the concentration of H3O+ ions. To determine the concentration of H3O+ ions, we need to rearrange the equation:
[H3O+] = \(10^-pH\) Substituting the value of pH =\(4.5×10^0\), we get: [H3O+] = \(10^-4.5\)
The value of \(10^-4.5\) can be calculated using scientific notation: \(10^-4.5\)= \(3.16×10^-5\) mol/L
for more such questions on concentration
https://brainly.com/question/28564792
#SPJ8
why does the ratio of chloride ions to calcium ions is 2:1 when calcium chloride forms
Answers
Sodium (Na) reacts with chlorine gas to form the ionic compound NaCl. Which of the following statements is true? A The reaction leaves each atom more unstable. B Sodium gains electrons from the chlorine to form the sodium ion. C Sodium ions are anions in the ionic bond. D By reacting, each atom fills its outer energy level with electrons.
Answers
Answer:
D
Explanation:
When Na and Cl react, Na gives up one electron to Cl to fill both their valence electron shells. Both atoms are more stable, chlorine gains the electrons, and chloride is the anion.
Answer: D. After reacting, each ion has a stable octet. (For Gradpoint)
Explanation:
A chemical reaction between X and Y forms C according to the reaction below. The data for three trials to measure the
rate of this reaction are also given.
Trial
1
2
3
[X] (M)
0.01
0.01
0.02
X+Y→C
[Y] (M)
0.015
0.030
0.015
What is the rate law for this reaction?
OR=KX²M
OR=KX³M²
OR=KXM²
OR=KX²M²
Initial Rate (M/s)
7.83x10-5
BIBE
3.13x 104
1.57x10
Answers
Explanation: The rate law for a chemical reaction is an equation that relates the rate of the reaction to the concentrations of the reactants. To determine the rate law for a reaction, experiments are typically conducted with different initial concentrations of the reactants and the initial rate of the reaction is measured.
From the data provided, it appears that the reaction is of the form X + Y → C. And the concentration of X and Y are varied in three trials and the corresponding Initial rate is measured.
In the first trial, [X] = 0.01 M and [Y] = 0.015 M, and the initial rate of the reaction is 7.83x10-5 M/s.
In the second trial, [X] = 0.01 M and [Y] = 0.03 M, and the initial rate of the reaction is 3.13x104 M/s.
In the third trial, [X] = 0.02 M and [Y] = 0.015 M, and the initial rate of the reaction is 1.57x10 M/s.
Given the data, the rate law for this reaction is OR = KX²M. This is because when the concentration of X is doubled, the rate of the reaction is quadrupled, which is consistent with a rate law of the form OR = k[X]^2.
Within a plant, photosynthesis is critical. It provides cells with the glucose they need for
energy. Photosynthesis is important for another reason, too. It's part of the flow of carbon
through organisms and the environment. This cycle, known as the carbon cycle, is essential
for life on Earth. It includes several processes that are occurring all the time. When plants
photosynthesize, carbon is removed from the atmosphere as carbon dioxide. When animals
and plants use cellular respiration, carbon is released into the atmosphere as carbon
dioxide. Organisms that die and are buried for millions of years turn into fossil fuels, which
are made of carbon. When humans burn those fossil fuels, carbon is released into the
atmosphere as carbon dioxide. Without enough carbon dioxide in the atmosphere, the
planet would be too cold. But too much carbon dioxide contributes to the warming of the
planet that could make it too hot to survive.
Based on the passage, what is one reason that the carbon cycle important?
A It adds carbon into the atmosphere whenever the level begins to drop too low.
B
It helps maintain a level of carbon in the atmosphere that is neither too hot nor
too cold.
с
It removes carbon from the atmosphere whenever the level begins to become
too high.
D
It releases carbon into space, preventing it from building up in Earth's
atmosphere.
Answers
Answer:
i think the answer is B
Explanation:
Answer:
B. (It helps maintain a level of carbon in the atmosphere that is neither too hot nor too cold.)
Explanation:
WHAT IS THE COLOR OF METHYL ORANGE
1. YELLOW
2. PINK
3. ORANGE
Answers
Answer:
3.Orange it literally say's it methyl ORANGE
Explanation:
Brainliest plz
Answer:
Other indicators
Indicator Acidic Alkaline
Methyl orange Red Yellow
Phenolphthalein Colourless Pink
The temperature of 100 grams of water changes from 16°C to 20°C. What is the total number of calories of heat energy absorbed by the water?
A.
25
B.
40
C.
100
D.
400
Answers
Answer:
D. 400 cal
Explanation:
Spec heat of water 1 cal/gm-C
1 cal / gm-C * 100 gm * ( 20-16 C) = 400 cal
cl-+peg=hcl+peg rate law, rate constant k
Answers
a. The rate law for this reaction is: Rate = k[Cl] [H₂]. This means that the rate of the reaction is directly proportional to the concentrations of both Cl and H₂ molecules.
What is rate law?Rate law is an equation that describes the rate of a chemical reaction as a function of the concentrations of reactants. The rate law allows us to describe how the rate of a reaction changes when the concentrations of reactants are changed. It is derived from the rate equation, which is a mathematical expression that can be used to calculate the rate of a reaction from the concentrations of the reactants and the rate constant.
b. The rate law for this reaction is: Rate = k[O] [Os]. This means that the rate of the reaction is directly proportional to the concentrations of both O and Os molecules.
c. The rate law for this reaction is: Rate = k[NO₂]₂. This means that the rate of the reaction is directly proportional to the square of the concentration of NO₂ molecules.
To learn more about rate law
https://brainly.com/question/16981791
#SPJ1
Complete Question:

Polymers are large organic molecules that are made of
a.
cations.
c.
carbon and oxygen only.
b.
anions.
d.
repeating units.
Answers
Answer:
D) Repeating units.
Explanation:
Polymers are large macromolecules which are made up of many repeating structural units called monomers :)
A bottle contains 75 mL of ethyl chloride. The density of ethyl chloride is 0.765 g/mL, what is the mass of ethyl chloride in the bottle?
Answers
Answer:
\(51y6. \times { \times }^{2} \)
What is the degree of oxidation of a simple substance
Answers
Answer: The oxidation state of a free element (uncombined element) is zero. For a simple (monoatomic) ion, the oxidation state is equal to the net charge on the ion. For example, Cl– has an oxidation state of -1. When present in most compounds, hydrogen has an oxidation state of +1 and oxygen an oxidation state of −2.
Hope this helps...... Stay safe and have a Merry Christmas!!!!!!!!!!! :D
Explanation:
the weak ionization constant (Ka) for HNO2 is equal to:

Answers
Answer:
the answer is A
Explanation:
The weak ionization constant (Ka) for HNO₂ is:
\(\displaystyle K_a = \frac{[H^+][NO_2^-]}{HNO_2}\)
What is the ionization constant?Acid-ionization constant Ka can be described as a quantitative measure of the strength of an acid in solution. It can be represented as the equilibrium constant for a chemical reaction:
\({\displaystyle {\ce {HA \longrightarrow A^- + H^+}}}\)
The chemical species HA can dissociate into A⁻ the conjugate base of the acid and a hydrogen ion, H⁺. In equilibrium, when the concentrations will not change over time, because both forward and backward reactions have the same rate.
The ionization constant can be described as the ratio of products and reactants raised to stoichiometric powers.
The dissociation constant is defined as:
\({\displaystyle K_{\text{a}}=\mathrm {\frac {[A^{-}][H^{+}]}{[HA]}} }\)
Given the dissociation of the HNO₂ as follows:
HNO₂ ⇄ H⁺ + NO₂⁻
The weak ionization constant (Ka) for HNO₂ is equal to:
\(\displaystyle K_a = \frac{[H^+][NO_2^-]}{[HNO_2]}\)
Therefore, option A is correct.
Learn more about ionization constant, here:
https://brainly.com/question/13794673
#SPJ2
According to the law of conservation of matter, we know that the total number of atoms does not change in a chemical reaction and
thus mass is conserved. Consider this model of a chemical reaction:
Reactants
Products
+ ?
The Substances
Undergoing Reaction
The Substances
Generated by the Reaction
How many oxygen molecules should be added to the reactants side to obey the law of conservation of matter?
Answers
Answer:
Compounds are represented by chemical formulas.
Explanation:
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
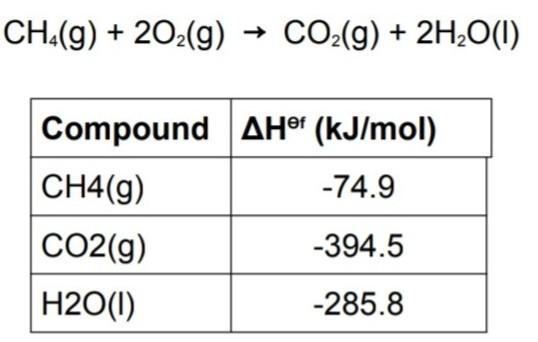
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8