Answers
Answer:
Circulatory
Explanation:
Related Questions
Solar and wind energy are both intermittent resources that cannot be relied upon for a constant stream of energy production. Explain why developing better ways to store energy is an important part of making these energy sources more practical to use.
Answers
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers.
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers. Energy storage's inherent ability to offer backup power in the event of grid failure is a feature that both residential consumers and commercial owners find highly desirable.
To know more about energy, here:
https://brainly.com/question/1932868
#SPJ1
Give and proidi the following after and undergoing alpha decay and beta decay
Answers
The products of the alpha decay of radium-226 and the beta decay of carbon-14 are radon-222 and nitrogen-14, respectively.
The alpha decay of radium-226 results in the emission of an alpha particle, which is a helium nucleus consisting of two protons and two neutrons.
Therefore, the product of the alpha decay of radium-226 is radon-222:
Ra-226 → Rn-222 + alpha particle
On the other hand, In the case of carbon-14, beta minus decay occurs, in which a neutron is converted into a proton, and an electron and an antineutrino are emitted.
So carbon-14 becomes nitrogen-14:
C-14 → N-14 + beta particle
To know more about alpha decay, here
brainly.com/question/27870937
#SPJ1
--The complete Question is, What is the product of the alpha decay of radium-226 and the beta decay of carbon-14?--
Are neutrons inside the nucleus ?
Answers
Neutrons and protons, commonly called nucleons, are bound together in the dense inner core of an atom, the nucleus, where they account for 99.9 percent of the atom's mass. ... The neutron was discovered in 1932 by the English physicist James Chadwick.
Hope this helps : )
Hope this helps!
Also please can I have the brainiest?!
the Na2O valence is
Answers
7. Which object is excellent at holding onto electrons?

Answers
Answer:
Insulator
Explanation:
if a student can run 5.5 mph, how long will it take the student to run 3.2 km
Answers
Answer: 13.5 minutes to run 3.2 km.
Explanation: To solve this problem, you need to convert the distance from kilometers to miles and the speed from miles per hour to kilometers per hour. 3.2 km is approximately 1.988 miles and 5.5 mph is approximately 8.851 kph. To find the time it takes to run 1.988 miles at 8.851 kph, you can use the formula time = distance ÷ speed. Plugging in the values, you get time = 1.988 miles ÷ 8.851 kph, which simplifies to approximately 0.225 hours or 13.5 minutes.
Therefore, it will take the student approximately 13.5 minutes to run 3.2 km.
A student drops a rubber ball from a height of 100cm and draws a diagram to show the path of a bouncing ball. Describe the energy transformation that occurs as the ball goes from position A to E. Include the words kinetic energy and potential energy in your explanation.
Picture is down below.

Answers
Answer:
hjgihgvoj
Explanation:
Apex Leaming-Pre-Lab
4. The graph shows a plot of the amount of a radioactive material remaining in
a sample versus time. According to the graph, what is the half-life of carbon-
14? Explain how you used the graph to determine this information.

Answers
The half-life of the carbon-14 obtained from the graphical representation of radioactive material remaining in a sample versus time is 5730 years.
How do i determine the half-life of carbon-14?The half-life a radioactive material is the time taken for half the material to decay or disintegrate.
For example, if the initial mass of a material is 10 g and the material becomes 5 g after 2 days. Then, we can say that the half-life of the material is 2 days.
With the above information in mind, we can obtain the half-life of the carbon-14 as illustrated below:
From the graph, we obtain:
Initial amount of carbon-14 = 1Half the initial amount = 1/2Now, we shall obtain the time for 1/2 in the graph.
The time for 1/2 in the graph is 5730 years.
Thus, we can conclude that the half-life of the carbon-14 is 5730 years.
Learn more about half life:
https://brainly.com/question/2279134
#SPJ1
46 g of glycerin were dissolved in 100 g of water. What is the freezing point of this solution?
Additional information:
М(С3Н5(ОН)3) = 92 g/mol;
Тf(Н2О) = 273.15 К;
Кf = 1.86 kg⋅К/mol.
Answers
Based on the formula to determine the freezing point depression of the solvent, the freezing point of the solution is 263.85 K.
What is the freezing point of a substance?
The freezing point of a substance is the temperature at which the liquid changes to solid without any further decrease in temperature occurring during the process.
The addition of solute substances in liquids usually lowers the freezing point of the liquid solvent.
The formula to determine the freezing point depression of solvent is given below:
ΔT = i * Kf * mwhere'
ΔT is the change in freezing point,i is the van't Hoff factor,Kf is the freezing point depression constant, andm is the molality of the solution.The molality of the given solution = moles of solute/kg of solvent
moles of solute = 46/92
mass of solvent = 100 g or 0.1 kg
Molality of solution = (46/92) / 0.1
Molality of solution = 5
for glycerine, i = 1
ΔT = ΔT = 1 * 1.86 * 5
ΔT = 9.3
The freezing point of the solution = 273.15 - 9.3
The freezing point of the solution = 263.85 K
Learn more about freezing point depression at: https://brainly.com/question/30093044
#SPJ1
hi. what's the electron number of He gas?
Answers
Answer:
Explanation:
2 electrons
Because the atomic number of helium is 2
WS Percent yield don’t understand how to do would appreciate the help

Answers
Answer:
1. Theoretical yield of NaOH is 22.72 g
2. Percentage yield of NaOH = 22.14%
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
NaHCO₃ —> NaOH + CO₂
From the balanced equation above,,
1 mole of NaHCO₃ decomposed to produce 1 mole (i.e 40 g) of NaOH and 1 mole (i.e 44.01 g) of CO₂.
Next, we shall determine the number of mole of NaHCO₃ that will decompose to produce 25 g of CO₂. This can be obtained as follow:
From the balanced equation above,,
1 mole of NaHCO₃ decomposed to produce 44.01 g of CO₂.
Therefore, Xmol of NaHCO₃ will decompose to 25 g of CO₂ i.e
Xmol of NaHCO₃ = 25 / 44.01
Xmol of NaHCO₃ = 0.568 mole
1. Determination of the theoretical yield of NaOH.
From the balanced equation above,,
1 mole of NaHCO₃ decomposed to produce 40 g of NaOH.
Therefore, 0.568 mole of NaHCO₃ will decompose to produce = 0.568 × 40 = 22.72 g of NaOH.
Thus, the theoretical yield of NaOH is 22.72 g
2. Determination of the percentage yield of NaOH.
Theoretical yield of NaOH = 22.72 g
Actual yield of NaOH = 5.03 g
Percentage yield of NaOH =?
Percentage yield = Actual yield /Theoretical yield × 100
Percentage yield = 5.03 / 22.72 × 100
Percentage yield of NaOH = 22.14%
someone help. trying to past this test but keep failing on this question.
Atoms may emit light energy when____
a. protons move to a higher energy level
b. protons move to a lower energy level
c. electrons move to a higher energy level
d. electrons move to a lower energy level
Answers
Answer:
When electrons move to a lower energy level.
Explanation:
4.The voltaic cell has with Pt/H+/H2 and Ag/AgC/Cl- half cells is a possible design for an electronic pH meter, in that the actual cell E depends on [H3O+].
(a) Write out (under each half cell) the electrode reactions, and give below the overall cell equation.
(b) Indicate with arrows the direction of motion of the ions and electrons as the cell reacts spontaneously.
(c) Mark the electrodes as + or – and cathode or anode.
(d) What is the standard cell potential, Eo?
Eo = _______________________
(e) Calculate the actual cell potential, E, if the unknown [H3O+] is 1.0 x 10-4 M.
E = _________________________
(f) If [H+] remains variable, then for this cell E = A + B.pH. What are the values of the Constants A and B?
A = ____________ , B = ______________
Answers
Answer:
(a) Electrode reactions:
Pt/H+/H2: 2H+(aq) + 2e- -> H2(g) (reduction)
Ag/AgCl/Cl-: AgCl(s) + e- -> Ag(s) + Cl-(aq) (reduction)
Overall cell equation: 2AgCl(s) + H2(g) -> 2Ag(s) + 2HCl(aq)
(b) Direction of motion of ions and electrons:
In the Pt/H+/H2 half-cell, hydrogen ions (H+) move towards the platinum electrode and accept electrons to form hydrogen gas (H2). In the Ag/AgCl/Cl- half-cell, silver ions (Ag+) move towards the silver chloride (AgCl) electrode and accept electrons to form silver (Ag) metal while chloride ions (Cl-) move away from the electrode. Electrons move from the hydrogen electrode to the silver electrode through the external circuit.
(c) Electrode labeling:
The Pt/H+/H2 electrode is the cathode (-) and the Ag/AgCl/Cl- electrode is the anode (+).
(d) Standard cell potential (Eo):
The standard cell potential can be calculated using the standard reduction potentials for each half-cell:
Eo(cell) = Eo(reduction, Ag/AgCl/Cl-) - Eo(reduction, Pt/H+/H2)
Eo(reduction, Ag/AgCl/Cl-) = +0.222 V (from standard reduction potential tables)
Eo(reduction, Pt/H+/H2) = 0 V (by definition)
Eo(cell) = +0.222 V - 0 V = +0.222 V
(e) Actual cell potential (E):
E(cell) = Eo(cell) - (0.0592 V / n) * log[H3O+]
where n is the number of electrons transferred in the balanced equation (2 in this case)
E(cell) = +0.222 V - (0.0592 V / 2) * log(1.0 x 10^-4 M)
E(cell) = +0.222 V - (0.0296 V) = +0.1924 V
(f) Values of constants A and B:
E(cell) = A + B.pH
At pH 7 (neutral), E(cell) = Eo(cell) = +0.222 V
Therefore, A = +0.222 V and B = -0.0592 V/pH
6. Which describes particles or electromagnetic radiation emitted from the nucleus during
radioactive decay ?
a) harmless nuclear fallout
b) nuclear radiation
c) transmutation
d) daughter nuclides
Answers
Answer:
A harmless nuclear fallout
Explanation:
just did this
Hydrogen and Bromine do not react when placed in a dark bottle, while it reacts explosively in a colourless transparent glass this is because
1. coloured glass reacts with bromine
2. coloured glass reacts with hydrogen
3. colourless transparent bottle activates the bromine hydrogen mixture
4. colourless glass permits the visible light to reach the reaction mixture
Answers
Hydrogen and Bromine do not react when placed in a dark bottle, while it reacts explosively in a colourless transparent glass this is because colourless glass permits the visible light to reach the reaction mixture. Therefore, the correct option is option 4.
Chemical reaction, the transformation of one or more chemicals (the reactants) into one or more distinct compounds (the products). Chemical elements or chemical compounds make up substances.
Chemical reactions constitute a fundamental component of life itself, as well as technology and culture. burning fuels, manufacturing glass and pottery, smelting iron, and brewing beer. Hydrogen and Bromine do not react when placed in a dark bottle, while it reacts explosively in a colourless transparent glass this is because colourless glass permits the visible light to reach the reaction mixture.
Therefore, the correct option is option 4.
To know more about Chemical reaction, here:
https://brainly.com/question/29039149
#SPJ1
Which statement below regarding evaporation is not correct?A. When a liquid is first placed in a closed container, the evaporation rate is higher than it is in an open container under the same conditions. B. When a liquid is first placed in a closed container, the evaporation rate is higher than the condensation rate. C. When the rates of evaporation and condensation are equal, the system has reached dynamic equilibrium. D. Vapor pressure refers to the pressure of a gas at a given temperature in equilibrium with its liquid phase. E. Stronger intermolecular forces within the liquid typically result in a lower vapor pressure under a given set of conditions.
Answers
Answer:
A. When a liquid is first placed in a closed container, the evaporation rate is higher than it is in an open container under the same conditions.
Explanation:
Evaporation refers to the process by which a liquid changes to gas or vapor. It usually occurs at the surface of a liquid.
Factors that affects evaporation include; nature of the liquid, surface area of liquid exposed, temperature and wind.
Considering the options given:
A. When a liquid is first placed in a closed container, the evaporation rate is higher than it is in an open container under the same conditions. = False
This is false because, the more the surface of the liquid exposed, the higher the rate of evaporation.
B. When a liquid is first placed in a closed container, the evaporation rate is higher than the condensation rate. = True
This is true because when a liquid is first placed in a container, the equilibrium position favors evaporation initially than condensation as saturation of the space above the liquid with vapor has not been achieved.
C. When the rates of evaporation and condensation are equal, the system has reached dynamic equilibrium.= True
Dynamic equilibrium is achieved when the rate of forward and backward reaction, in this case, evaporation and condensation are equal.
D. Vapor pressure refers to the pressure of a gas at a given temperature in equilibrium with its liquid phase. = True
E. Stronger intermolecular forces within the liquid typically result in a lower vapor pressure under a given set of conditions. = True
Strong intermolecular forces between the molecules of the liquid causes the liquid to remain in the liquid state rather than turning to vapor, thereby lowering vapor pressure
AIP+Na2S=AI2S3+Na3P? What’s the balance equation?
Answers
The balanced chemical equation of the AIP+Na₂S=AI₂S₃+Na₃P is given as 2 AlP + 3 Na₂S = Al₂S₃ + 2 Na₃P.
Where:
AlP = Aluminum Phosphide
Na₂S = Sodium Sulfide
Al₂S₃ = Aluminum Sulfide
Na₃P = Sodium Phosphide
The balanced chemical equation to produce aluminum sulfide (Al₂S₃) and sodium phosphide (Na₃P) from the reaction between aluminum phosphide (AlP) and sodium sulfide (Na₂S) is given as,
2 AlP + 3 Na₂S = Al₂S₃ + 2 Na₃P
Where the reaction between 2 molecules of AlP and 3 molecules of Na₂S gives the 2 molecules of Al,3 molecules of sulfide, 5 molecules of Sodium, and 2 molecules of phosphide Na₃P.
To learn more about balanced chemical equations:
https://brainly.com/question/26694427
#SPJ1
While isobaric heat can be measured by using the coffee cup calorimeter, what kind of device would be needed to measure the reaction heat under isochoric condition? Please search literature to answer the question.
To measure the reaction heat more accurately at isobaric condition, what modification(s) would you suggest making on the coffee cup calorimeter? Please justify the suggested change(s).
Answers
To measure reaction heat under isochoric conditions, a bomb calorimeter is needed.
This device is designed to maintain a constant volume (isochoric) during the reaction, allowing for accurate measurement of reaction heat. To improve the accuracy of the coffee cup calorimeter for measuring reaction heat under isobaric conditions, a modification that could be made is to use a stirring device to ensure uniform mixing of the reactants and to minimize heat loss to the surroundings.
Additionally, a lid with a small hole could be placed over the top of the calorimeter to prevent heat loss while still allowing for pressure equalization. These modifications would help to minimize errors in heat measurement and improve the accuracy of the results obtained.
To know more about the Calorimeter, here
https://brainly.com/question/24150308
#SPJ1
Please help i will give brainliest!!! thank you =)
A. 1
B. 2
C. 3
B. no coefficient is needed

Answers
Answer:
Answer choice B. 2
Explanation:
What is rate of reaction?
Answers
Answer:
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place. Reaction rate is defined as the speed at which reactants are converted into products. Reaction rates can vary dramatically.
Explanation:
PLS MAKE ME AS BRAINLIST
The powerful, warm, fast Atlantic Ocean current is called the
A.Atlantic Curren
B.Rip Tide
C.Gulf Stream
D.El Nino
60 points!
Answers
Answer:
C
Explanation:
Warm water is heated by the Gulf Stream, a warm air current that originates in the Gulf of Mexico. As the warm water moves north, it forces cooler water to sink and move south.
the reason of which I do not believe it is a Rip Tide is because rip tides have been found in both several rivers and oceans, if not all.
Use this information to calculate AGxn-
2C₂H₂(g) +50₂(g) →4CO₂(g) + 2H₂O(g)
AGCH₂=209.2 kJ/mol
AGE.co=-394.4 kJ/mol
AGHO-228.57 kJ/mol
AG
=
DONE
2,453.1
-1,616.7
-2,453.1
kJ
Answers
-1,616.7 kJ/mol
2C₂H₂(g) +50₂(g) →4CO₂(g) + 2H₂O(g)
AGC₂H₂=209.2 kJ/mol
AGE.co2=-394.4 kJ/mol
AG H2O-228.57 kJ/mol
AG 5O2 = ?
Now From the above given equation , we have got the enthalpy of no. of moles in reaction
4 moles of CO₂ contains = 4 -394.4 kJ/mol = -1577.6
2 moles of H₂O contains = 2 -228.57 kJ/mol = - 457
2 moles of C₂H₂ contains = 2 209.2 kJ/mol = +418.4
now putting all the values in equation
50₂(g) = 4CO₂(g) + 2H₂O(g) - 2C₂H₂(g)
50₂(g) = -1577.6-457-418
50₂(g) = -1,616.7 kJ/mol
The amount of heat that a system releases or absorbs during a chemical reaction is known as the enthalpy change. The symbol for it is HEnthalpy is measured in the same SI unit as energy, the joule. At constant pressure, the expression known as "enthalpy change" is computed or determined. It is impossible to measure a system's absolute enthalpy. As a result, the enthalpy change is measured. In endothermic processes, the change in enthalpy is positive, while in exothermic reactions, it is negative.To know more about Enthalpy visit : https://brainly.com/question/13775366
#SPJ9
Need help with chemistry study guide. Just need question 1 answered

Answers
There are 4 main factors that affect the rate of a chemical reaction:
1. Concentration of reactants, if you have more reactants, the reaction must produce more products in order to reach equilibrium
2. Surface area, the bigger the surface area, the more collisions can occur and therefore the reaction will occur in a higher rate
3. Temperature, if you increase the temperature this means an increase of kinetic energy, which is movement, therefore with more movement, more collisions and more reaction occurring
4. Catalyst, this is a substance that the main function is to lower the energy of activation of a reaction, therefore it will increase the rate of the reaction
A
B
C
D
an atom has a diameter of 500 pm. Calculate the number of atoms that have been lined up to form a 50 cm long line.
Answers
The number of atoms that will form the given line is 1 x 10⁹ atoms.
number of atoms that forms the given lengthThe number of atoms that has formed the given length is calculated as follows;
number of atoms x diameter of one atom = length of the line
The given parameters include;
diameter of an atom = 500 pm = 500 x 10⁻¹² m
Length of the line = 50 cm = 0.5 m
The number of atoms that will form the given line is calculated as follows;
number of atoms = 0.5 m / 500 x 10⁻¹² m
number of atoms = 1 x 10⁹ atoms
Thus, the number of atoms that will form the given line is 1 x 10⁹ atoms.
Learn more about number of atoms here: https://brainly.com/question/6258301
#SPJ1
what are all the ways that a substance can change state?
Answers
Explanation:
hdhehdbrhdns dhdjdjdhrjs dhfirjr rudjdbe dbdud d
Answer:
Matter can change from one state to another if heated or cooled. If ice (a solid) is heated it changes to water (a liquid). This change is called MELTING. If water is heated, it changes to steam (a gas).
Explanation:
A sample of helium is initially at 535 torr in a volume of 2.85 L.
If this sample of helium is at 24.7 °C, then What quantity in moles of
helium are present?
Answers
Answer:
There are 0.082 moles of He present.
Explanation:
You would use the equation PV=nRT for this problem.
P (must convert to atm) = 535 torr/ 0.704 atm
V = 2.85 L
n = being solved for
R= 0.082 (universal gas constant for atm)
T (must convert to Kelvin) = 24.7 celsius/ 297.85 K
This equals:
0.704(2.85)= n (0.082)(297.85)
2.0064 =24.42n
n= 0.082 moles
Why do the balls of the Newton’s cradle eventually stop?
Answers
The balls of the Newton’s cradle eventually stop because they loose energy as they collide.
The balls of the Newton’s cradle lose energy to the air as they move through it due to friction.
These balls make sound when they collide, and consequently the balls loose energy to heat upon collision.
The balls loose energy as they collide with each other and eventually stop.
Thus, we can conclude that the balls of the Newton’s cradle eventually stop because they loose energy as they collide.
Learn more about Newton’s cradle here: https://brainly.com/question/14063949
Which of these organisms can move on its own?
A oak tree
B anteater
C dolphin
D all of the above
Answers
Element is defined as either a
Answers
Answer:
An element is an ATOM or aggregate of atoms all of which have the same number of protons. In pure form, they will also have the same number of electrons. Each element has its own unique number of protons. For example, hydrogen always has one proton, helium always has two and lithium always has three.
Explanation:
How much energy is contained in the six-cookie serving size recommended on the label?
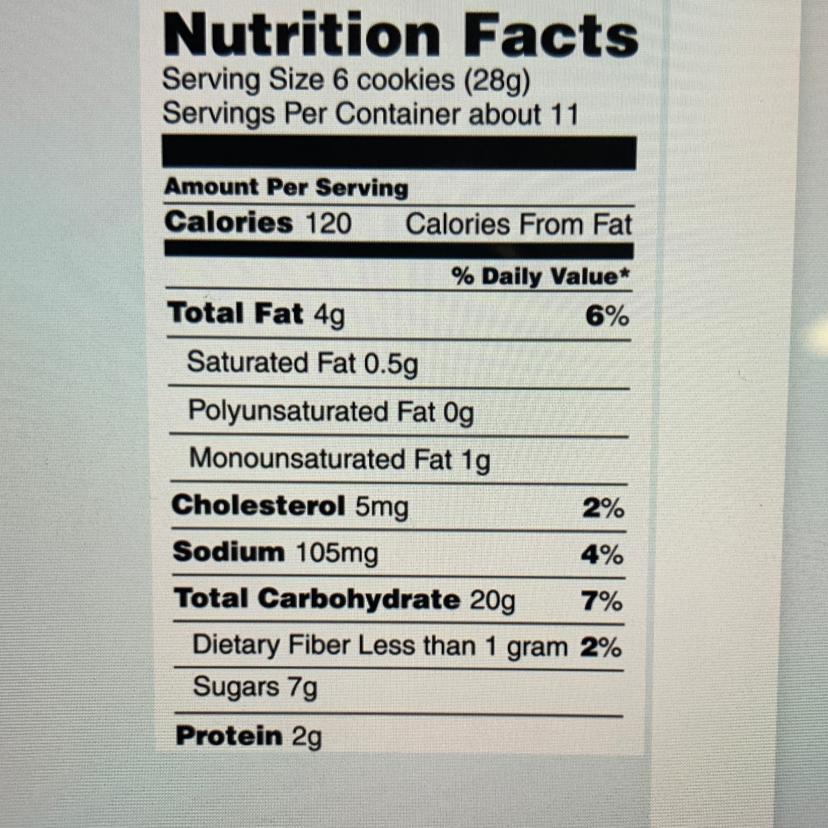
Answers
Monounsaturated Fat 1g