According to the law of conservation of mass, mass cannot be gained or destroyed in a chemical reaction. Why can't you simply add the masses of two reactants to determine the total mass of product? Choose the best answer and explain.a. One of the reactants could be present in excess, and not all of it will be used to make the product(s).b. The masses of the reactants must be converted to moles first and then added.c. Not all chemical reactions follow the law of conservation of mass, especially ones with mixed physical states present.d. The masses of the two reactants cannot be added until they are each multiplied by their coefficient in the balanced equation.e. It is only the molar masses that are conserved in chemical reactions, not the actual mass amounts given in the laboratory.
Answers
The correct answer is d.) The masses of the two reactants cannot be added until they are each multiplied by their coefficient in the balanced equation.
This is because the law of conservation of mass only applies when the reactants and products are in their balanced equation form. In order to determine the total mass of a product, the law of conservation of mass states that the mass of the reactants must be multiplied by the coefficients of the reactants in the balanced equation. This is because the coefficients represent the amount of each reactant or product present in the reaction, and the total mass of the product must be equal to the total mass of the reactants. Therefore, the masses of the two reactants cannot be added until they are each multiplied by their coefficient in the balanced equation.
To learn more about mass click here https://brainly.com/question/30388313
#SPJ4
Related Questions
What happens to valence electrons during a covalent bond?
Answers
Answer:
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. By sharing their outer most (valence) electrons, atoms can fill up their outer electron shell and gain stability.
The liver is a group of tissues that produce bile that is used to break down fats. The liver is a(n) _____.
Answers
Answer:
Its food for cannibals.
Why are elements in the tall columns of the periodic table called representative elements
Answers
Answer:
In addition, the number of valence electrons present in the elements of these groups denotes the group. The valence electrons present in their elements reflect their particular group, which is why they are termed representative elements.
Explanation:
When scientists analyze the fossil record, they find organisms that are similar to some organisms of today with noticeable changes. How does this help scientists to interpret the past? A. It doesn't help them at all. B. It allows them to see how organisms have changed over time. C. It allows them to see how organisms have remained the same over time. D. It allows them to see that there is no relationship between the past and today.
Answers
Answer:
The answer is B.
Explanation:
The logical explanation is that fossils from animals are changing over time caused by enviroment change ,natural disaters,astronmical events,and so on.These organisms need to adapt over time and so there features change.For Example the dragon fly used to be as big as a bus now that it has adapted and changed from when the cretaceous period it is as small as your index finger.
What happens when an electron absorbs photons? OA. It changes into a neutron and falls into the nucleus. B. It moves to a higher energy level. OC. It changes into an alpha particle and is ejected from the atom. OD. It falls to a lower energy level.
Answers
When an electron absorbs photons it moves to a higher energy level.
What happens when an electron absorbs photons?
Absorption of photons occurs when electrons in a given state absorb photons which causes them to gain energy and jump to higher energy levels.
The electrons will move to higher energy level because they have acquired more energy.
Thus, when an electron absorbs photons it moves to a higher energy level.
Learn more about energy level here: https://brainly.com/question/14287666
#SPJ1
what is the name of this branched alkane?

Answers
Answer:
Explanation:
3-methyl pentane.
Answer:
the answer is methyl pentane
What is the pKa of the side chain of histidine?
Answers
The pKa of the side chain of histidine is approximately 6.0. Histidine is an amino acid with an imidazole side chain.
This side chain consists of a nitrogen atom bonded to two hydrogen atoms, with a double bond connecting the nitrogen and a single bond connecting it to a carbon atom. This means that in aqueous solutions, the side chain of histidine is mostly in the form of a protonated (positively charged) species at a pH below 6.0 and mostly in the form of a deprotonated (negatively charged) species at a pH above 6.0. This is due to the fact that histidine has a carboxylic acid group (COOH) on the side chain, which is capable of donating a proton (H+) when the pH is low, and accepting a proton when the pH is high.
To learn more about histidine click here https://brainly.com/question/30599919
#SPJ11
Metal lons are typically _______
their corresponding neutral atoms. Nonmetalons are typically ________
than their
corresponding neutral atoms.
Answers
Metal ions are typically smaller than their corresponding neutral atoms. Nonmetal ions are typically larger than their corresponding neutral atoms.
When a metal atom loses one or more electrons to become an ion, the number of protons in the nucleus remains the same while the number of electrons decreases. This results in a stronger attraction between the remaining electrons and the nucleus, causing the electron cloud to contract and the ion to become smaller.
For example, a neutral sodium atom (Na) has 11 electrons, while a sodium ion (Na+) has lost one electron and has only 10 electrons. The 10 electrons are still attracted to the same 11 protons in the nucleus, resulting in a smaller ion.
On the other hand, when a nonmetal atom gains one or more electrons to become an ion, the number of protons in the nucleus remains the same while the number of electrons increases. This results in a weaker attraction between the electrons and the nucleus, causing the electron cloud to expand and the ion to become larger.
For example, a neutral chlorine atom (Cl) has 17 electrons, while a chloride ion (Cl-) has gained one electron and has 18 electrons. The 18 electrons are not as strongly attracted to the 17 protons in the nucleus, resulting in a larger ion.
Metal ions are smaller than their corresponding neutral atoms because the loss of electrons causes the electron cloud to contract. Nonmetal ions are larger than their corresponding neutral atoms because the gain of electrons causes the electron cloud to expand.
To know more about Metal, visit;
https://brainly.com/question/4701542
#SPJ11
Can somebody list only 2 ways whales are different from fish?
(Make it only like 1 sentence)
(WILL MARK BRAINLIEST)
:D
Answers
Answer:
1. whales are mammals and fish are not
2. Fish have gills which extract oxygen from the water and thus allow it to live underwater its entire life. Whales on the other hand do not have gills but instead have one or two blowholes
3. whales provide milk to their young and fish do not!
Explanation:
Check off each one that shows a correct conversion. Example: 1 mol Al= 6.022 x 10^23 atoms Al would be checked off.
A. 2.5 mol CaCO3 = 1.51 x 10^24 molecules CaCO3
B. 2 mol Al2O3 = 101.96 g Al2O3
C. 1 mol Be3N2 = 55.06 g

Answers
Answer:
Explanation:
A. molecules of CaCO3 = number of moles x avogadros number
= 2.5 x 6.022 x 10^-23 = 1.5055 x 10^-22
B. the mass of 2 moles of Al2O3 is = molar mass of Al2O3 x number of moles
the molar mass of Al2O3 = 2 x molar mass of Al + 3 x molar mass of O
= 2 x 27 + 3 x 16 = 102 g/mol
the mass of Al2O3 = 102 x 2 = 204 g
C. 1 mol of Be3N2 = number of moles x molar mass of Be3N2
molar mass of Be3N2 = 3 x molar mass of Be + 2 x molar mass of N
= 3 x 9 + 2 x 14 = 55g/ mol
so the mass of 1 mol of Be3N2 is 55 g
What is the molarity (M) of 0.6 mol NaOH in 0.250 L of solution?
Answers
Molarity (M) is a unit of concentration used in chemistry. It is defined as the number of moles of solute dissolved in one liter (L) of solution. The unit of molarity is expressed as moles per liter (mol/L) or M.
Molarity is commonly used to describe the concentration of solutions. It is particularly useful in performing calculations involving chemical reactions, dilutions, and stoichiometry.
Given information,
Moles of solute = 0.6 mol
The volume of solution = 0.250L
The formula of molarity is:
Molarity (M) = Moles of solute / Volume of solution
Molarity (M) = 0.6/0.250
Molarity (M) = 2.4 M
Therefore, the molarity of the solution is 2.4 M.
Learn more about molarity, here:
https://brainly.com/question/2817451
#SPJ1
what determines electrical charge of polyatomic ions
Answers
Answer: Polyatomic ions each carry a specific charge, which is determined by their numbers of valence electrons.
Enter the balanced net ionic equation for the potentially unbalanced equation HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+KCl(aq)HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+KCl(aq).
Express your answer as a chemical equation. Identify all of the phases in your answer.
Answers
The balanced net ionic equation for the potentially unbalanced equation:
HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+KCl(aq)
HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+KCl(aq) is as follows:
Net Ionic Equation:
H+ (aq) + CO32- (aq) → H2O (l) + CO2 (g)
The complete ionic equation of the above-mentioned reaction is:
H+ (aq) + Cl- (aq) + 2K+ (aq) + CO32- (aq) → 2K+ (aq) + Cl- (aq) + H2O (l) + CO2 (g)
Now let us understand the chemical reaction involved in the given equation:
HCl(aq) + K2CO3(aq) → H2O(l) + CO2(g) + KCl(aq)
The given chemical reaction is a double replacement reaction in which hydrochloric acid reacts with potassium carbonate to give water, carbon dioxide, and potassium chloride.
The balanced net ionic equation for the potentially unbalanced equation:
HCl(aq)+K2CO3(aq)→H2O(l)+CO2(g)+KCl(aq) is H+ (aq) + CO32- (aq) → H2O (l) + CO2 (g).
In the complete ionic equation of the reaction, all the ions in the reaction are written separately. However, in the net ionic equation, only those ions that take part in the reaction are considered.
The balanced net ionic equation of the reaction:
HCl(aq) + K2CO3(aq) → H2O(l) + CO2(g) + KCl(aq) is the equation that represents the complete ionic equation by canceling out the spectator ions present on both sides of the equation. Therefore, the balanced net ionic equation for the given reaction is:
H+ (aq) + CO32- (aq) → H2O (l) + CO2 (g).
In summary, the balanced net ionic equation of the reaction HCl(aq) + K2CO3(aq) → H2O(l) + CO2(g) + KCl(aq) is H+ (aq) + CO32- (aq) → H2O (l) + CO2 (g). This equation represents the complete ionic equation by canceling out the spectator ions present on both sides of the equation.
To know more about ionic equation visit:
brainly.com/question/29299745
#SPJ11
Determine the ph of a 1. 0 l buffer prepared by adding 0. 100 moles of naoh to 0. 250 moles of hf. ka for hf = 3. 5 × 10−4
Answers
The pH of a 1. 0 l buffer prepared by adding 0. 100 moles of NaOH to 0. 250 moles of HF. ka for HF = 3. 5 × 10−4 is 3.86.
What is Henderson hasselbalch equation?The Henderson hasselbalch equation can be expressed as
pH = pKa + log [base]/ [acid]
Firstly we will calculate the value of pKa
pKa = -logKa
Given,
Ka = 3. 5 × 10−4)
pKa = -log(3. 5 × 10−4)
pKa = 3.46
Now, we will calculate the value of log [base]/ [acid]
Given,
[base] = 0.10m
[acid] = 0.25m
log [base]/ [acid] = log(0.10/0.25)
= 0.4
Putting the values in handerson hasselbalch equation,
pH = 3.46+0.4
= 3.86
Thus, we find that the value of pH of a 1. 0 l buffer prepared by adding 0. 100 moles of NaOH to 0. 250 moles of HF is 3.86.
learn more about pH:
https://brainly.com/question/15052769
#SPJ4
Which process is not chemical weathering?
A. Acid Rain hitting rocks
B. Cave water (with minerals) breaking down sandstone
C. Salt water flowing over rocks
D. Ice breaking a rock
Answers
Answer:
D because there's no minerals its just the ice
Explanation:
Answer:
Ice breaking a rock
Explanation:

consider a solution made from a nonvolatile... consider a solution made from a nonvolatile solute and a volatile solvent. which statement is true? multiple choice the vapor pressure of the solution is always greater than the vapor pressure of the pure solvent. the boiling point of the solution is always greater than the boiling point of the pure solvent. the freezing point of the solution is always greater than the freezing point of the pure solvent.
Answers
The correct statement is "the boiling point of the solution is always greater than the boiling point of the pure solvent." When a nonvolatile solute is added to a volatile solvent, the vapor pressure of the resulting solution is lower than that of the pure solvent.
This is known as vapor pressure depression. As a result, the boiling point of the solution is raised above that of the pure solvent, as more energy is required to reach the higher boiling point. Similarly, the freezing point of the solution is lowered below that of the pure solvent, as the nonvolatile solute disrupts the formation of the pure solvent's crystal lattice.
This is known as freezing point depression. Therefore, the correct option is the second statement: "the boiling point of the solution is always greater than the boiling point of the pure solvent."
To know more about vapor pressure click this link -
brainly.com/question/26127294
#SPJ11
Explain the role of a primary consumer and a secondary consumer in a food web.
Answers
Answer:
Explanation:
Secondary consumers are organisms that eat primary consumers for energy. Primary consumers are always herbivores, or organisms that only eat autotrophic plants.
Carnivores only eat other animals, and omnivores eat both plant and animal matter.
formula for lead(III) sulfite?
Answers
Answer:
PBSO4
FOrmula of lead(III) sulfite
PBSO4
A 220.0 mL sample of helium gas is in a cylinder with a movable piston at 105 kPa and 275 K. The piston is
pushed in until the sample has a volume of 95.0 mL. The new temperature of the gas is 310 K. What is the new
pressure of the sample?
274 kPa
216 kPa
Piston
243 kPa
51.1 kPa

Answers
Answer:
Explanation:
what did you get?
A reptile egg is shown in the diagram.
Y
Which best describes the function of the structure
labeled Y?
O food source
O protection
waste collection
gas exchange
HELP

Answers
Answer:Its D
Explanation:
Trust
Answer:
food source
Explanation:
Got 100%
Select the correct answer. Which of the below is an example of mimicry that enables prey species avoid predation
Answers
What are the answers? I assume you meant to attach a photo.
Answer:
Thus, the one that is an example of mimicry that enables prey species to avoid predation is a harmless organism imitating the look of a harmful one (option B).
Explanation:
qual volumes of two monoprotic acid solutions ( a and b ) are titrated with identical naoh solutions. the volume to reach the equivalence point for solution a is twice the volume required to reach the equivalence point for solution b , and the ph at the equivalence point of solution b is higher than the ph at the equivalence point for solution a . which statement is true?
Answers
Statement that is true is : D). Solution A has more concentrated acid than the solution B and acid in solution A is weaker acid than that in solution B.
Why solution A has more concentrated acid than B?The last statement is true and states that a solution A is comparatively more concentrated than solution B but the acid present in solution A is feeble.
This is because that solution A is excessively concentrated which requires an extra NaOH to attain the point of equivalence.
And as the pH that exists at the point of equivalence point is more for solution A, therefore it implies that solution A carries a weaker acid.
To know more about equivalence point, refer
https://brainly.com/question/23502649
#SPJ4
Note: The question given on portal is incomplete. Here is the complete question.
Question: Equal volumes of two monoprotic acid solutions (A and B) are titrated with identical NaOH solutions. The volume to reach the equivalence point for solution A is twice the volume required to reach the equivalence point for solution B, and the pH at the equivalence point of solution A is higher than the pH at the equivalence point for solution B. Which statement is true?
A) The acid in solution A is less concentrated than in solution B and is also a weaker acid than that in solution B.
B) The acid in solution A is more concentrated than in solution B and is also a stronger acid than that in solution B.
C) The acid in solution A is less concentrated than in solution B and is also a stronger acid than that in solution B.
D) The acid in solution A is more concentrated than in solution B and is also a weaker acid than that in solution B.
microscale organic laboratory with multistep and multiscale syntheses dana w. mayo, ronald m. pike, and david c. forbes
Answers
The "Microscale Organic Laboratory with Multistep and Multiscale Syntheses" is a valuable resource for students studying organic chemistry. It offers a practical approach to learning and reinforces important concepts through hands-on experimentation.
The book titled "Microscale Organic Laboratory with Multistep and Multiscale Syntheses" is written by Dana W. Mayo, Ronald M. Pike, and David C. Forbes. This book is designed to provide students with practical experience in conducting organic chemistry experiments on a small scale.
In the book, the authors emphasize the importance of understanding and mastering multistep and multiscale syntheses. These syntheses involve a series of reactions that are carried out in a step-by-step manner to achieve a desired final product. This approach allows students to develop their skills in planning and executing complex organic reactions.
One of the advantages of conducting experiments on a microscale is that it requires smaller amounts of chemicals and reduces waste. This makes the experiments safer, more cost-effective, and environmentally friendly. Additionally, working on a smaller scale allows for quicker reactions and easier purification of products.
The book includes detailed instructions and procedures for a variety of organic chemistry experiments. It covers a wide range of topics, such as functional group transformations, stereochemistry, and organic synthesis. Each experiment is carefully explained, with clear step-by-step instructions and explanations of the underlying principles.
By working through the experiments in this book, students can gain a deeper understanding of organic chemistry concepts and develop essential laboratory skills. The book also provides examples of common mistakes and troubleshooting tips, which can help students improve their technique and achieve better results.
Learn more about organic chemistry here:-
https://brainly.com/question/9477180
#SPJ11
A substance that gives up a proton during a chemical reaction, raising the hydrogen ion concentration of water, is most appropriately called.
Answers
A substance that gives up a proton during a chemical reaction, raising the hydrogen ion concentration of water, is most appropriately called acid.
What is an acid?An acid is a substance that produces hydrogen ion as the only positive ion produced in water. This is the Arrhenius definition of an acid. As such, the acid gives out a proton to water and oxonium ion is formed.
Thus, substance that gives up a proton during a chemical reaction, raising the hydrogen ion concentration of water, is most appropriately called acid.
Learn more about acid:https://brainly.com/question/14072179
#SPJ4
Think about multiplying each speed by a factor to calculate kinetic energy at that speed. Is there a common factor that works for every speed? If so, what’s this factor?
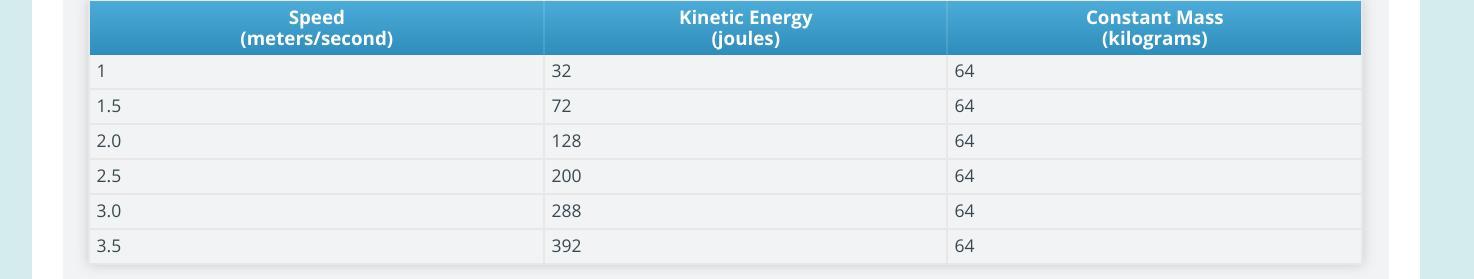
Answers
Answer:
No, there’s no common factor that works for every speed.
Explanation:
A sample containing 37. 4 grams of ammonia undergoes combustion with excess oxygen in a bomb (constant volume) calorimeter to form nitrogen monoxide (nitric oxide) and water gas. The heat constant for the calorimeter is 952 J/oC. The specific heat capacity for water vapor is 2. 00 J/ g. OC. The specific heat capacity for nitrogen monoxide is 0. 996 J/ g. OC. During the experiment, the temperature in the calorimeter changes from 22. 9oC to 1772. 9oC.
A. Give the balanced chemical equation that represents this reaction and calculate the number of grams for each product produced.
B. Calculate the amount of heat transferred during this reaction. Be certain to include units and the correct number of significant figures. Ignore the effects of excess oxygen in the bomb calorimeter.
C. Calculate the change in enthalpy in kJ/mol ammonia for this reaction.
D. Is the change in enthalpy equal to the change in internal energy for this reaction? Explain your answer.
E. How would the change in enthalpy for this reaction differ, if the reaction was allowed to occur in an open reaction vessel? Explain
Answers
There is a production of 59.14 grammes of water vapour and 65.85 grammes of nitrogen monoxide.
A. The balanced chemical equation for the combustion of ammonia with excess oxygen to form nitrogen monoxide and water gas is:
4 NH₃ (g) + 5 O₂ (g) → 4 NO (g) + 6 H₂O (g)
According to the equation, for every 4 moles of ammonia, 4 moles of nitrogen monoxide and 6 moles of water gas are produced. To calculate the number of grams of each product produced, we need to find the number of moles of ammonia in the sample:
Molar mass of NH₃ = 14.01 g/mol + 3(1.01 g/mol) = 17.04 g/mol
Number of moles of NH₃ = 37.4 g / 17.04 g/mol = 2.19 mol
Using the mole ratio from the balanced equation, we can calculate the number of moles of each product:
Number of moles of NO = 4/4 x 2.19 mol = 2.19 mol
Number of moles of H₂O = 6/4 x 2.19 mol = 3.28 mol
To find the mass of each product, we can use their molar masses:
Molar mass of NO = 30.01 g/mol
Molar mass of H₂O = 18.02 g/mol
Mass of NO produced = 2.19 mol x 30.01 g/mol = 65.85 g
Mass of H₂O produced = 3.28 mol x 18.02 g/mol = 59.14 g
Therefore, 65.85 grams of nitrogen monoxide and 59.14 grams of water gas are produced.
B. The amount of heat transferred during this reaction can be calculated using the formula:
q = CΔT
where q is the amount of heat transferred, C is the heat capacity of the calorimeter, and ΔT is the change in temperature of the calorimeter.
From the problem, we are given:
C = 952 J/°C
ΔT = 1750°C - 22.9°C = 1727.1°C
Substituting the values, we get:
q = (952 J/°C) x (1727.1°C) = 1.64 x \(10^{6}\) J
Therefore, the amount of heat transferred during this reaction is 1.64 x \(10^{6}\) J.
C. The change in enthalpy for the reaction can be calculated using the equation:
ΔH = q/n
where q is the amount of heat transferred, and n is the number of moles of ammonia that reacted.
From part A, we know that 2.19 moles of ammonia reacted. Substituting the values, we get:
ΔH = (1.64 x \(10^{6}\) J) / 2.19 mol = 748,858 J/mol = 748.9 kJ/mol
Therefore, the change in enthalpy for the reaction is 748.9 kJ/mol ammonia.
D. The change in enthalpy is not equal to the change in internal energy for this reaction because the bomb calorimeter is a constant volume calorimeter, which means that no work is done during the reaction (Δw = 0). Therefore, the change in enthalpy (ΔH) equals the change in internal energy (ΔU) plus the work done (Δw) by the system:
ΔH = ΔU + Δw
Since Δw = 0 for the bomb calorimeter, ΔH = ΔU.
To learn more about balanced chemical equation refer to:
brainly.com/question/28294176
#SPJ4
In calculating the mass of an atom the mass of the electrons is essentially
Answers
Answer:
Sex
Explanation:
A sample of grape juice has a hydroxide ion concentration of 1.4 × 10-10 M. Which of these equations will you use to find the hydronium ion concentration?
Answers
Explanation:
Since [H+] [OH-] =10^(-14)
[H+] = (10^-14) / (1.4×10^-10)
Que representa en la reacción química
Na2SO4(s)+H2O(1)=
Answers
Answer: The given reaction is a double displacement reaction.
Explanation:
A chemical reaction equation in which both positive and negative ions of two different reactants are exchanged then it is called a double displacement reaction.
For example, \(Na_{2}SO_{4} + H_{2}O \rightarrow H_{2}SO_{4} + Na_{2}O\)
Here, hydrogen ions of \(H_{2}O\) are replaced by the sodium ions of \(Na_{2}SO_{4}\). Also, sulfate ion of \(Na_{2}SO_{4}\) are replaced by oxygen ion of \(H_{2}O\).
Thus, we can conclude that the given reaction is a double displacement reaction.
3. Convert 273.0 g of lead into moles.
Answers
Answer:
1.3175675675675604
Explanation:
does this need to be rounded?