According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? The volume and the shape stay the same. The volume increases to fill the new container, but the shape stays the same. The volume stays the same, but the shape changes to fit the new container. The volume and the shape change to fill the new container.
Answers
Answer:
C, "The volume stays the same, but the shape changes to fit the new container."
Explanation:
i took the test & got this question correct.
Answer:
It's C
Explanation:
For Edge:)
Related Questions
What are the best practices while sharing a lab balance with other students?.
Answers
The best practices while sharing a lab balance with other students are cleaning if dirt is present it is needed to be cleaned as it will introduce errors in the measurements.
What are errors?Errors in chemical analysis result when there is a difference between observed value and the true value.If the magnitude of errors is large , it results in decrease in accuracy, reproducibility, and precision.
There are three types of errors:1) random error 2) systematic error 3) human error.The cause of random errors are difficult to quantify while the human errors can be minimized by taking a range of readings to reduce the error.
Errors while measuring boiling point may be human errors while noting down the boiling temperature or instrumental or systematic error if there is a fault in the thermometer.
Learn more about errors,here:
https://brainly.com/question/15810279
#SPJ2
plzzzzz help me I will mark u brainiest

Answers
Answer:
Both 1 and 2 is the correct answer
Answer:
both 1 and 2
Explanation:
they are maleable and conduct electricity due to the layers of positive ions being able to move
now make this the branliest answer
PLEASE HELP!!!
How many km/hr are there in 6.01 x 10^6 yd/wk?
Answers
Answer:
32.711
Explanation:
1 yard = 0.0009144 km
1 week = 168 hrs
6.01 x 10^6 / 168 = 35,773.81 yds/hr
= 32.711 km/hr
what is the industrial application of nitrogen monoxide
Answers
Answer:
it can be in the production of nitric acid
Answer:
Explanation:
Nitrogen oxides are used in the production of nitric acid, lacquers, dyes, and other chemicals. Nitrogen oxides are also used in rocket fuels, nitration of organic chemicals, and the manufacture of explosives.
when the n quantum number equals 1 we are in what orbital
A)d
B) f
C)p
D)s
Answers
i think it is D i hope this helps yall
Salt is considered to be an ionic substance, while sugar is commonly thought of as a
covalent (or molecular) substance. Keeping that in mind, if you were given 2
unidentified white crystals and asked to classify them as either ionic or covalent in
nature, what steps would you take and what evidence would you look for?
Answers
To classify two white crystals as either ionic or covalent in nature, you could perform a series of tests and observations to gather evidence and make a determination.
what are the steps that are followed ?
Solubility: Ionic substances tend to dissolve readily in water, forming a clear solution, while covalent substances are often insoluble in water and form a cloudy solution. You could dissolve a small amount of each crystal in water and observe the solution.
Conductivity: Ionic substances are good conductors of electricity in aqueous solution, while covalent substances are poor conductors. You could measure the conductivity of the solutions from step 1.
Melting and boiling points: Ionic substances have high melting and boiling points due to the strong electrostatic forces between ions, while covalent substances have lower melting and boiling points due to weaker intermolecular forces. You could measure the melting and boiling points of the crystals.
Crystal structure: Ionic substances form a crystal lattice structure due to the repeating arrangement of ions, while covalent substances often have a more random molecular arrangement. You could observe the crystal structure of the substances under a microscope.
By performing these tests and observations, you should be able to gather enough evidence to classify the two white crystals as either ionic or covalent in nature.
To learn more about covalent substances follow the given link: https://brainly.com/question/29551714
#SPJ1
what is the molarity of the solution if the mole fraction of ethanol in water is 0.2 ?
Answers
Answer: 2.1M
Explanation:
Hence, the molarity of a solution of ethanol in water is 2.1M.
Which two features of Earth's crust involve tension?
O A. Convergent boundaries
B. Reverse faults
c. Divergent boundaries
O D. Normal faults
Answers
Answer:
The correct options are;
C. Divergent plate boundary
D. Normal faults
Explanation:
Tensile stress tends to pull objects part by acting axially upon the object to pull the object on a cross section perpendicular to the objects cross-section
The most common stress in convergent plate boundaries is compression stress
The most common stress in divergent plate boundaries is tensile stress
In the presence of tensile stress, normal faults results in the raising of mountains due to their enormous forces
Therefore, the features of Earth's crust involving tension are divergent plate boundary and normal faults.
Answer:c and d
Explanation:
The mass In grams of fluorine in a sample of MgF 2 that contains 20.1 grams of magnesium is:A)10.6 grams B)6.4 grams C)42.4 grams D)16.5 grams
Answers
1. In order to answer this one and the next one, we will use mass percentage as our guide, since every compound has a mass percentage for each element, and this is found by checking the total mass and the separate masses of each element, for example, MgF2, the mass percentage is 39% for Mg and 61% for F2, this is due to their molar mass, 24g for Mg and 38g for F2
We have 27.1 g of Mg, and this will always correspond for 39% of the total mass of the compound, so we can do the following math:
100% = x
39% = 27.1g
x = 69.5 grams is the total mass of MgF2, now we only have to subtract the value of Mg and then we will have F2
69.5 - 27.1 = 42.4 grams, this is the mass of Fluorine
2. The same step by step from the previous question, 36% of the total mass is Al and 64% is S, but this question has a little difference, now we have the mass of the compound, but the process is very similar
100% = 50.2 grams
64% = x grams
x = 32.1 grams, which can be considered as letter A
how many grams of water (18.016 g/mol) are theoretically produced for the following reaction given we have 2.6 moles of hcl and 1.4 moles of ca(oh)2?
Answers
The total grams is 46.8g which is option C. The correct answer;
2HCl + Ca(OH)2 -------------> CaCl2 + 2H2O
2 moles HCl reacts with 1 mole Ca(OH)2
2.6 moles of HCl reacts with 2.6 x 1 / 2 = 1.3
but we have 1.4 moles Ca(OH)2
so Ca(OH)2 is exess reagent
HCl is limiting reagent.
2 mole HCl gives 2 moles of H2O
2.6 moles of HCl gives 2.6 moles of H2O
convert moles to mass
moles = mass / molar mass
mass = moles x molar mass
mass = 2.6 x 18 = 46.8 g
answer = option c = 46.8 g
To know more about moles, visit;
brainly.com/question/26416088
#SPJ4
Ammonium chloride, ammonium sulfate and ammonium nitrate are used in fertilisers.
Ammonia reacts with nitric acid to make ammonium nitrate.
NH3 + HNO3 → NH4NO3
Calculate the mass of ammonia required to make 5.0 g of ammonium nitrate.
relative formula mass of NH3 = 17
relative formula mass of NH4NO3 = 80
Answers
Answer:
1.0625 g
Explanation:
Number of moles in 5g of NH4NO3 = 5.0g/80g/mol = 0.0625 moles
Again the balanced reaction equation is;
NH3 + HNO3 → NH4NO3
So,
1 mole of NH3 yields 1 mole of NH4NO3
x moles of NH3 yields 0.0625 moles of NH4NO3
x = 0.0625 moles of NH3
Mass of NH3 = number of moles * molar mass
Mass of NH3 = 0.0625 moles * 17g/mol
Mass of NH3 = 1.0625 g
i’m too dumb for school.
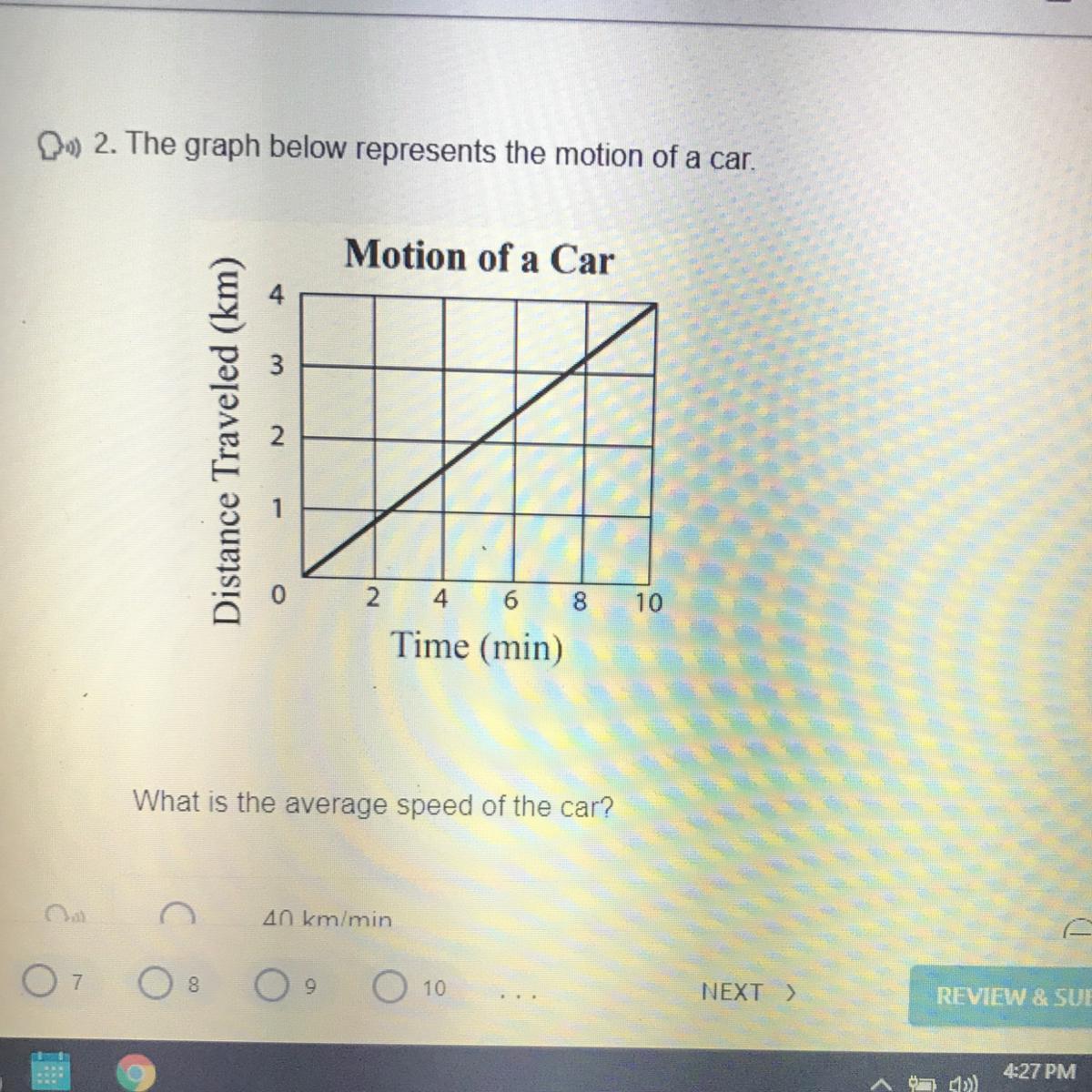
Answers
Answer:
every two minutes the car moves 1km
Explanation:
Consider the isotope: mn-54. How many protons are in the atom of mn-54 if the atom has a charge of +3?.
Answers
Even though the atom of Mn-54 has a charge of +3, the atom contains 25 protons. The atomic number of an atom is also determined by the proton number.
What is protons?A proton is a stable subatomic particle with the symbols p, H+, or 1H+ and an electric charge of +1 e. Its mass is 1836 times greater than that of an electron and only slightly less than that of a neutron. A proton, a subatomic particle, is found in the nucleus of every atom. The particle has an electrical charge that is positive and opposite to the electron's.
What is a protons made of and can we destroy protons?There are two up quarks and one down quark in protons. Neutrons are made up of one up quark and two down quarks. The "strong nuclear force," one of the four fundamental forces, holds the nucleus together (gravity and electromagnetism are two others).
The law of energy conservation states that matter cannot be created or destroyed. As a result, an atom cannot be destroyed or divided into smaller particles. The three fundamental particles that make up an atom are the electron, proton, and neutron.
Briefing:Without changing the type of atom or element, the proton number of an element's atoms will never vary. In other words, all isotopes of an element have the same atomic number, which is also known as proton number. The proton number stays constant even when the atoms lose some electrons.
The atomic number of manganese (Mn) is 25. It has 25 protons, so. Even if the Mn atoms are charged, this proton count stays the same.
To know more about Proton visit:
https://brainly.com/question/1252435
#SPJ4
How would Dr. Eijkman test his new hypothesis?
Answers
Dr. Eijkman can test his new hypothesis by designing and conducting experiments that aim to investigate the relationship between certain factors and the observed phenomenon. These experiments can involve controlled variables, data collection, statistical analysis, and comparison with existing knowledge.
To test his new hypothesis, Dr. Eijkman would first design an experimental setup that allows him to manipulate and control the variables relevant to his hypothesis. He would choose a suitable sample size and experimental conditions to ensure reliable results. The specific details of the experiment would depend on the nature of his hypothesis and the phenomenon under investigation.
Dr. Eijkman would then conduct the experiment, carefully following the procedures and recording relevant data. This could involve measuring certain parameters, observing changes over time, or conducting comparative studies. The collected data would be analyzed using appropriate statistical methods to determine if there is a significant relationship or correlation supporting his hypothesis.
The results of the experiment would be compared with existing knowledge and previous studies in the field to validate or refine the hypothesis. Dr. Eijkman would also consider potential limitations or confounding factors that might affect the interpretation of the results. The process of testing the hypothesis may involve multiple iterations of experiments, data analysis, and refinement of the experimental design until conclusive results are obtained.
Learn more about statistical analysis here:
https://brainly.com/question/30154483
#SPJ11
40. 0% carbon, 6. 7% hydrogen, and 53. 3% oxygen with a molecular mass of 60. 0 g/mol. What is the molecular formula of the unknown compound?
Answers
The molecular formula of the unknown compound is C2H2O2.
To determine the molecular formula of the unknown compound, we need to calculate the empirical formula first and then find the multiple of its subscripts to obtain the molecular formula.
Given:
Percentage of carbon = 40.0%
Percentage of hydrogen = 6.7%
Percentage of oxygen = 53.3%
Molecular mass = 60.0 g/mol
Step 1: Convert the percentages to grams.
Assuming we have 100 grams of the compound:
Mass of carbon = 40.0 g
Mass of hydrogen = 6.7 g
Mass of oxygen = 53.3 g
Step 2: Convert the masses to moles using the molar masses of the elements.
Molar mass of carbon = 12.01 g/mol
Molar mass of hydrogen = 1.008 g/mol
Molar mass of oxygen = 16.00 g/mol
Number of moles of carbon = Mass of carbon / Molar mass of carbon
= 40.0 g / 12.01 g/mol
= 3.332 mol
Number of moles of hydrogen = Mass of hydrogen / Molar mass of hydrogen
= 6.7 g / 1.008 g/mol
= 6.648 mol
Number of moles of oxygen = Mass of oxygen / Molar mass of oxygen
= 53.3 g / 16.00 g/mol
= 3.331 mol
Step 3: Determine the empirical formula by dividing the moles by the smallest value.
Dividing the moles of carbon, hydrogen, and oxygen by 3.331 gives approximately 1 for each element.
So, the empirical formula of the compound is CHO.
Step 4: Determine the multiple of the subscripts to obtain the molecular formula.
To find the multiple, we divide the molecular mass by the empirical formula mass.
Molecular mass = 60.0 g/mol
Empirical formula mass = (12.01 g/mol) + (1.008 g/mol) + (16.00 g/mol) = 29.018 g/mol
Multiple = Molecular mass / Empirical formula mass
= 60.0 g/mol / 29.018 g/mol
= 2.07
Rounding to the nearest whole number, we get 2.
Therefore, the molecular formula of the unknown compound is C2H2O2.
learn more about molecular here
https://brainly.com/question/30640129
#SPJ11
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
If we look at a molecule of water, H2O, we see there are 3 atoms in each molecule. So, we can see that 1 mole of water contains 3 moles of atoms (2 moles of H, 1 mole of O). Therefore, water contains 6.02 × 1023 atoms/mole × 3, or 1.81 × 1024 atoms. If that is true, how many atoms are in 1.00 mole of glucose, C6H12O6?
URGANT
Answers
Answer:
1 molecule of glucose contains 6 atoms of C, 12 atoms of H, and 6 atoms of O • 1 mole of glucose contains 6 moles of C atoms, 12 moles of H atoms, and 6 moles of O atoms.
Can I get Brainliest?
Explanation:
Why is science important?
Answers
Answer:
science is valued by the society because the application of scientific knowledge helps to satisfy many basic human needs and improve living standards
Explanation:
Finding a cure for cancer and a clean form of energy are just two typical examples
Answer:
Science is about a whole lot more than that and to sum it up we believe that science is a way of helping the brain grow in finding new knowledge and helps us defeat our curiosity of how the world develops and works today. Science is important because it has helped form the world that we live in today.
Explanation:
In other words, science is one of the most important channels of knowledge. It has a specific role, as well as a variety of functions for the benefit of our society: creating new knowledge, improving education, and increasing the quality of our lives. Science must respond to societal needs and global challenges.
w h y d I d t h I s t a k e m e s o l o n g
I was wondering that like whenever I boil milk I just have to keep standing in front of it to make sure that I don't waste any milk but then I was wondering like Why does it even overflow ? In case of water it doesn't happen. What makes it to behave like this ?
Answers
Answer: Milk is an emulsion of fat and water. When you boil milk, the fat separates to the top and forms a layer on top.
how do water molecules undergo physical change as they move from the solid to the gaseous state?
Answers
In the solid state, water molecules are bound together in an orderly, structured lattice.
What is gaseous state?Gaseous state is a physical state in which matter exists as a gas. In this state, particles are very far apart and have no definite shape or volume. Examples of gaseous states include steam, oxygen, and nitrogen. Gases are typically very compressible and can occupy a wide range of volumes and pressures.
When heated, the molecules break the intermolecular bonds and move randomly, becoming a liquid. As the water is heated further, the molecules move faster and faster and eventually break apart into individual atoms. This process is called evaporation, and it occurs when the molecules have enough energy to break away from the liquid surface and move into the atmosphere. In the gaseous state, the water molecules are no longer bound together and are free to move independently
To know more about gaseous state click-
https://brainly.com/question/1776161
#SPJ4
compare the following: acid 1: hypochlorous acid , hclo acid 2: hydrogen sulfide ion , hs- acid 3: hydrofluoric acid , hf what is the formula for the strongest acid ?
Answers
The formula for the strongest acid is: HF.
To compare the strengths of the given acids, we can use their acid dissociation constants (Ka). The larger the Ka value, the stronger the acid.
The Ka values for the given acids are:
Hypochlorous acid (HClO): Ka = 3.5 × 10^-8
Hydrogen sulfide ion (HS^-): Ka = 1.0 × 10^-7
Hydrofluoric acid (HF): Ka = 6.8 × 10^-4
Comparing the Ka values, we can see that hydrofluoric acid (HF) has the largest Ka value and is therefore the strongest acid among the three given acids.
The formula for the strongest acid is: HF.
To know more about hydrofluoric acid refer here:
https://brainly.com/question/24194581
#SPJ11
11
When an object is dropped into a graduated cylinder, the water in the
cylinder rises from 50.OmL to 56.3 mL. The mass of the object is 15.9 g.
What is the density of the object? *
Answers
Answer:
The answer is 2.52 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question
mass of object = 15.9 g
volume = final volume of water - initial volume of water
volume = 56.3 - 50 = 6.3 mL
It's density is
\(density = \frac{15.9}{6.3} \\ = 2.523809...\)
We have the final answer as
2.52 g/mLHope this helps you
if i have a compound that is composed of 6.00 g of carbon, and 2.01 g of hydrogen, what is its formula?
Answers
The formula of a compound composed of 6.00 g of carbon and 2.01 g of hydrogen is CH₄.
The atomic weight of carbon is 12.01 g/mol, whereas hydrogen's atomic weight is 1.01 g/mol.
The number of moles of carbon present in the compound = 6.00 g ÷ 12.01 g/mol = 0.499 mol.
The number of moles of hydrogen present in the compound = 2.01 g ÷ 1.01 g/mol = 1.99 mol.
To make the quantity of carbon and hydrogen equal, both quantities should be divided by 0.499. As a result, the following ratio is obtained:-
Carbon: 0.499 mol/0.499 mol = 1
Hydrogen: 1.99 mol/0.499 mol = 4
The ratio of carbon to hydrogen is 1:4, which corresponds to the formula CH₄. Thus, the formula of the compound is CH₄.
Learn more about compound: https://brainly.com/question/704297
#SPJ11
Jen is walking her dog at a constant rate.
They keep a constant rate as they turn a
corner. Why has their velocity changed?

Answers
Answer:
4is the answer
Explanation:
given that benzaldehyde is a meta- director, in the same marvin editor draw all three resonance structures for the carbocation intermediate that results from step 2 in the electrophilic aromatic substitution reaction when benzaldehyde reacts with br2 in the presence of febr3. if you do not remember the structure of the benzene derivative, consult the l3 complete lecture notes slides
Answers
In the electrophilic aromatic substitution reaction between benzaldehyde and Br2 in the presence of FeBr3, the first step involves the generation of a carbocation intermediate. This carbocation is formed when the bromine molecule attacks the benzene ring, displacing a proton.
Since benzaldehyde is a meta-director, the carbocation intermediate will be stabilized through resonance. The resonance structures can be represented as follows:
Structure 1:
Br
|
Ph-C(+)-H
|
Structure 2:
Br
|
Ph-C-H
| |
+ Ph
Structure 3:
Br
|
Ph-C-H
| |
Ph +
In these resonance structures, the positive charge of the carbocation is delocalized throughout the benzene ring. The presence of the electron-withdrawing aldehyde group (CHO) in benzaldehyde directs the incoming bromine atom to the meta position relative to the aldehyde group.
Please note that it's always recommended to consult reliable sources and appropriate references for accurate structural representations.
To know more about carbocation visit:
https://brainly.com/question/31827291
#SPJ11
on the first day of your new job as a chemist, you are given a bottle of magnesium sulfate and asked to make 30 ml of 0.3 m mgso4. the formula on the bottle is mgso4∗7h2o (also k
Answers
Using stoichiometry and the molar mass, we find that one would need approximately 2218.32 milligrams of MgSO₄·7H₂O (Epsom salt) to make 30 mL of 0.3 M MgSO₄ solution.
The molar mass of MgSO₄·7H₂O is calculated as follows:
MgSO₄ : (24.31 g/mol + 32.06 g/mol + (4 × 16.00 g/mol)) = 120.37 g/molH₂O : (2 × 1.01 g/mol + 16.00 g/mol) = 18.02 g/molTherefore, the molar mass of MgSO₄·7H₂O is :
(120.37 g/mol + (7 × 18.02 g/mol)) = 246.48 g/mol30 mL of 0.3 M MgSO₄ solution is prepared by finding the amount of MgSO₄·7H₂O required in milligrams:
0.3 moles/L × 0.03 L = 0.009 moles0.009 moles × 246.48 g/mol = 2.21832 gramsConverting grams to milligrams, multiplying by 1000:
2.21832 grams × 1000 = 2218.32 milligramsTherefore, to make 30 mL of 0.3 M MgSO₄ solution, you would need approximately 2218.32 milligrams of MgSO₄·7H₂O.
To know more about stoichiometry, refer to the link :
https://brainly.com/question/28780091#
#SPJ11
Complete question :
On the first day of your new job as a chemist, you are given a bottle of magnesium sulfate and asked to make 30 mL of 0.3 M MgSO4. The formula on the bottle is MgSO4∗7H2O (also known as Epsom salt). Calculate the amount of salt you need (in milligrams).
which term best described energy stored in batteries and food
Answers
Answer:
energy stored is potential.
Explanation:
2. How many mi hr is 30km/s?
Answers
The answer is 67 108.0888km/s.
What do winston and julia incorrectly believe to be true as they promise to work for the brotherhood?
Answers
Winston and Julia incorrectly believe that they will be able to overthrow the Party and establish a better society through their work for the Brotherhood.
Throughout the novel "1984" by George Orwell, Winston and Julia are disillusioned with the oppressive Party and the society it has created. They both believe that they can make a difference and change things for the better. As they promise to work for the Brotherhood, a secret organization dedicated to overthrowing the Party and establish , they are convinced that they will be able to achieve their goals and create a free and equal society.
However, their beliefs are based on a misunderstanding of the Party's power and the extent of its control over society. They underestimate the Party's ability to manipulate and suppress dissent, and they fail to recognize the true nature of the Brotherhood and its leader, Emmanuel Goldstein. In the end, their efforts are in vain, and they are captured and subjected to the Party's brutal methods of torture and brainwashing.
To know more about establish visit:
https://brainly.com/question/28646672
#SPJ11
describe the chemistry and main ingredients of uv gels
Answers
UV gels are commonly used in the nail industry for artificial nail enhancements. The main ingredients in UV gels are typically oligomers, monomers, photo initiators, and pigments.
Oligomers are long-chain molecules that provide the bulk and strength to the gel. Monomers are smaller molecules that help the gel cure and harden under UV light. Photoinitiators are added to the gel to initiate the polymerization reaction when exposed to UV light. This reaction causes the gel to harden and bond to the natural nail or nail extension. Pigments are added to give the gel its color and opacity.
The chemistry of UV gels involves the process of polymerization, which is the bonding of monomers and oligomers through a chemical reaction. This reaction is triggered by the photoinitiators in the gel when exposed to UV light. As the reaction occurs, the gel becomes solid and adheres to the nail.
Overall, the chemistry and ingredients of UV gels allow for a durable and long-lasting nail enhancement that is popular in the beauty industry.
To know more about pigments refer here :
https://brainly.com/question/14354023
#SPJ11