Answers
Answer:
Abraham thought that GOD would raise Issac back from the dead. It was a test and they would sacrifice the donkey instead.
Related Questions
What are the correct coefficients when this equation is balanced?
S+ O₂ → SO3
a. 2, 3, 2
b. 2, 6, 2
c. 1, 3, 2
d. 2, 1, 1
Answers
The correct coefficients for balancing the equation S + O₂ → SO₃ are: 2, 6, 2.option b.
In order to balance the equation, we need to ensure that the number of atoms of each element is the same on both sides of the equation. Let's break down the elements in the equation:
On the left side, we have:
- Sulfur (S): 1 atom
- Oxygen (O): 2 atoms
On the right side, we have:
- Sulfur (S): 1 atom
- Oxygen (O): 3 atoms
To balance the sulfur atoms, we need 2 molecules of SO₃ on the right side. This gives us:
- Sulfur (S): 2 atoms
- Oxygen (O): 6 atoms
Now, both sides have the same number of sulfur and oxygen atoms. Therefore, the balanced equation is: 2S + 3O₂ → 2SO₃. The correct coefficients are: 2, 6, 2, which corresponds to option b.
By multiplying the sulfur (S) coefficient by 2, we balance the sulfur atoms. Then, by multiplying the oxygen (O₂) coefficient by 3, we ensure that both sides have the same number of oxygen atoms.
The resulting coefficients are 2S + 3O₂ → 2SO₃. This balanced equation follows the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.option b.
for such more questions on coefficients
https://brainly.com/question/26322576
#SPJ8
A sample of gas is in a container with a movable piston. The volume in the container is originally 850 ML at a temperature of 467K and a pressure of 11 point 4K PA. What will the new temperature if the volume is expanded to 1125 in El with a new pressure of 99.7 K PA?
Answers
If the volume is increased to 1125 in El with a new pressure of 99.7 K PA, the new temperature will be around 808 K.
What transpires to the gas volume in a moveable piston cylinder?Once the piston's pressure has doubled, it goes downward until the gas's pressure and the piston pressure are equal. The gas has now lost half of its original volume. The volume of gas falls to one-fourth of its initial volume if the pressure on the piston is once more increased by a factor of two.
This issue can be resolved using the coupled gas law:
(P1V1) / T1 = (P2V2) / T2
Using the following conversions, we can first change the starting volume to litres and the original pressure to atmospheres (atm):
1 mL = 0.001 L
1 kPa = 0.00987 atm
V1 = 850 mL = 0.85 L
P1 = 11.4 kPa = 0.1126 atm
T1 = 467 K
The new volume and pressure can also be converted to litres and atmospheres:
V2 = 1125 mL = 1.125 L
P2 = 99.7 kPa = 0.984 atm
Now we can plug in the values and solve for T2:
(P1V1) / T1 = (P2V2) / T2
(0.1126 atm * 0.85 L) / 467 K = (0.984 atm * 1.125 L) / T2
T2 = (0.984 atm * 1.125 L * 467 K) / (0.1126 atm * 0.85 L)
T2 = 808 K
To know more about temperature visit:-
https://brainly.com/question/13294753
#SPJ1
Answer:
548 K
I hope this helps! Cheers ^^
Chlorine is heated with oxygen to form dichlorine monoxide gas. Express your answer as a chemical equation. Identify all of the phases in your answer.
Answers
Answer: 4 HCl (g) + O₂ (g) → 2 Cl₂ (g) + 2 H₂O (l)
Explanation:
4 moles of hydrogen chloride (note that it is in the gaseous phase, otherwise it would be hydrochloric acid) react with 1 mole of oxygen gas to form 2 moles of chlorine gas and 2 moles of liquid water.
To conform with the law of conservation of mass, the equation must be balanced, this means that there must be the same number of each type of atom on both sides of the arrow.
Is this helpful?
The balanced chemical reaction is:
4 HCl (g) + O₂ (g) → 2 Cl₂ (g) + 2 H₂O (l)
In first place, chlorine is heated with oxygen to form dichlorine monoxide gas. Then, this can be expressed as:
HCl (g) + O₂ (g) → Cl₂ (g) + H₂O (l)
On the other side, the Law of Conservation of Matter is also called the law of conservation of mass or the Law of Lomonósov-Lavoisier. This law postulates that "The mass is not created or destroyed, only transformed."
Then, the number of atoms that are present in the reagents has to be equal to the number of atoms present in the products.
Finally, the balanced chemical reaction is:
4 HCl (g) + O₂ (g) → 2 Cl₂ (g) + 2 H₂O (l)
Learn more:
https://brainly.com/question/15400776?referrer=searchResultshttps://brainly.com/question/13911443?referrer=searchResultshttps://brainly.com/question/13979405https://brainly.com/question/24209700?referrer=searchResultsNadia runs from her house to a fiend's house that is 24 meters away. How much time she will take to reach her friend's house, knowing that Nadia's speed is 3 m/s .
Answers
Nadia will take 8 seconds to reach her friend's house.
Speed is the measure of the distance traveled by an object per unit of time. It is a scalar quantity and is typically expressed in units such as meters per second (m/s), miles per hour (mph), or kilometers per hour (km/h).
To calculate the time Nadia will take to reach her friend's house, we can use the formula;
time = distance / speed
where distance is the amount of space traveled by an object, and time is the duration of travel.
Put the values given in the problem, we have:
time = 24 meters / 3 m/s
time = 8 seconds
Therefore, Nadia will take 8 seconds.
To know more about time here
https://brainly.com/question/15356513
#SPJ1
Cassini has a mass of 2523 kg, and Saturn
has a mass of 5.68 x 1026 kg. Saturn's radius
is 54,364 km. If Cassini feels a gravitational
force of 2.980 x 104 N, how high above
Saturn's surface is it?
Rearrange F gravity Gm,m₂/r2
to solve this problem
In 10 words or fewer, how high above Saturn's surface is the Cassini
satellite?
Answers
The F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and height is 108,728 km.
What is gravity?Gravity is the amount of force that is produced by the earth to attract the object toward the surface and it doubles if the mass is double.
The height of Saturn is the duble of the radius of the given radius of 54,364 km of the planet Saturn which is 108,728 km.
Therefore, F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and hight is 108,728 km.
Learn more about gravity, here:
https://brainly.com/question/4783082
#SPJ1
2- The vapor pressure of water at 80°C is 355 torr, Calculate the vapor
pressure of an aqueous solution made by dissolving 50 gm of ethylene
glycol (C₂H5O₂) in 50 gm of water. What is the vapor pressure lowering of
water in this solution?
Answers
The vapor pressure lowering of water in this solution is 1165 torr.
What is vapor pressure?
The pressure that a vapour exerts on its condensed phases in a closed system at a specific temperature is known as the vapour pressure or equilibrium vapour pressure. An indicator of a liquid's evaporation rate is the equilibrium vapour pressure. It has to do with how easily liquid-borne particles tend to elude detection (or solid). Volatile is a term used to describe a chemical that, at room temperature, has a high vapour pressure. Vapor pressure is the force that a vapour cloud exerts over a liquid surface. The kinetic energy of a liquid's molecules increases along with the temperature. The number of molecules vaporising also rises as a function of the molecules' rising kinetic energy, which raises the vapour pressure.
the vapor pressure of water and the mixture is 355 torr and 760 torrs. Therefore, the value of vapor pressure of oxygen would be,
Ptotal=PO2+PH2O
760=PO2+355
760−355=PO2
405=PO2
So, at the temperature, the vapor pressure of Ethylene is 405 torr.
When volume reduces to 50ml, the pressure of the oxygen would be,
P1V1=P2V2
where P1 and P2 are the pressure and V1 and V2 are the volumes.
P1V1=P2V2
P1V1/V2=P2
405×100 / 50=P2
810=P2
So, when the volume is 50ml the vapor pressure of the oxygen is 810 torr.
Now the total pressure of water-saturated oxygen at 50 ml volume is,
Ptotal=PO2+PH2O
Ptotal=810+355
Ptotal=1165
So, the total vapor pressure is 1165 torr.
To learn more about vapor pressure from given link
https://brainly.com/question/4463307
#SPJ9
What is a form of electromagnetic energy, the vibration of electrically charged particles
Answers
Answer:
Glossary: Electromagnetic wave. Definition: Electromagnetic radiation is generated by the vibration of electrons or other electrically charged particles
Explanation:
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
The stage in which the hair begins to destroy itself as it disconnects from the
papilla is called?
Answers
PLEASE HELP IMMEDIATELY I NEED THE ANSWER NOT A HINT THANK YOU
Answers
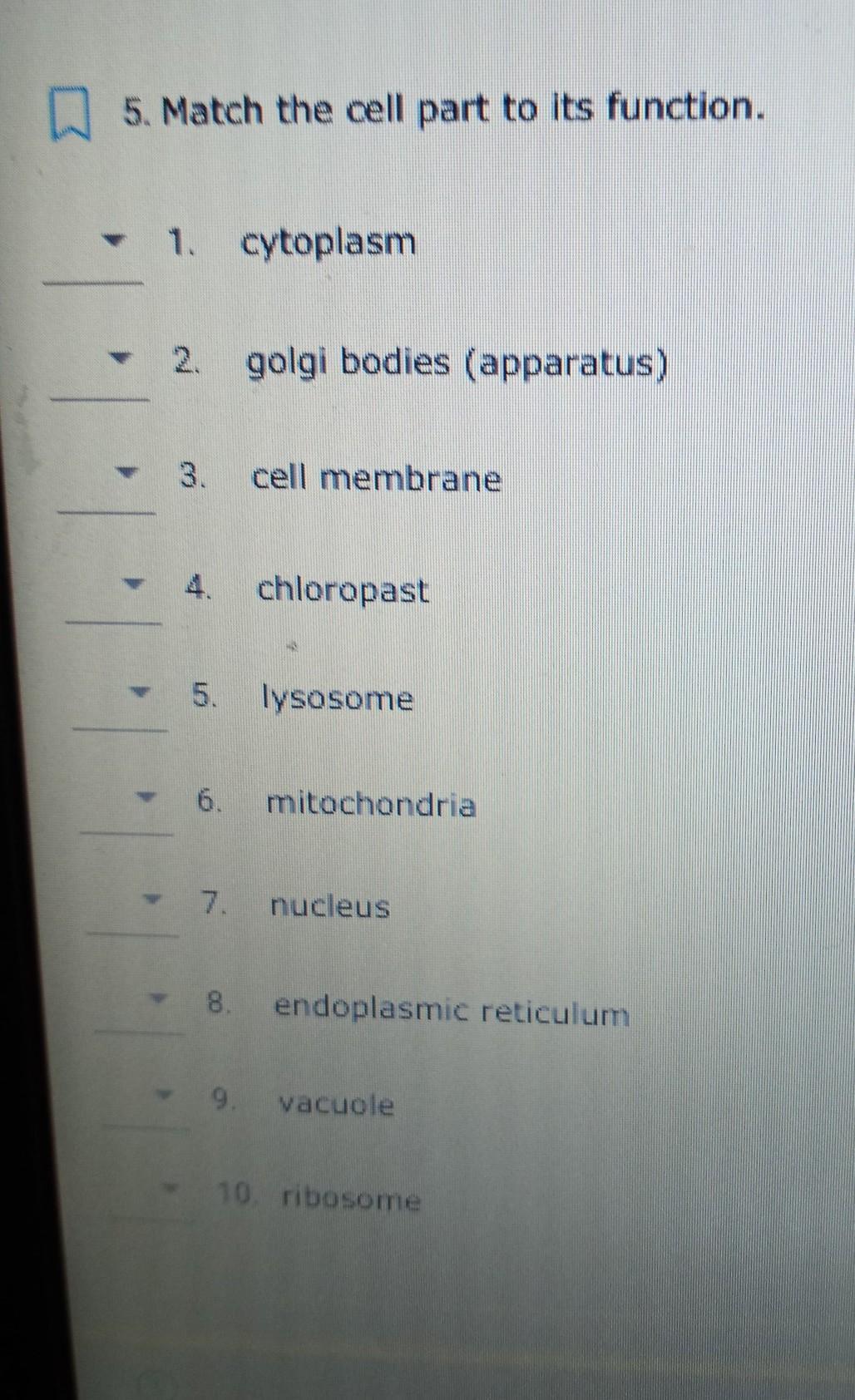
Cytoplasm: gel like environment which allows organelles to move about the cell
Golgi bodies: packages and ships materials out of the cell
Cell membrane: controls what goes in and out of the cell
Chloroplast: makes food for plant cells using sunlight
Lysosome: breaks down waste, food, and worn out cell parts
Mitochondria: breaks down food to release energy for the cell
Nucleus: contains the cell's DNA and is the control center of the cell
Endoplasmic reticulum: transports materials within cell; process lipids
Vacuole: stores water, waste and food
Ribosome: make proteins
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
The vapor pressure of benzene at 25°C is 95.1 mm Hg and of toluene 28.4 mm Hg. The mass fractions of benzene and toluene in a solution are 0.5.
a) Calculate the partial pressure of benzene and toluene above the solution.
b) Calculate the total vapor pressure above the solution.
c) Calculate the vapor composition above the solution.
Answers
Answer:
df dffdffdfd dffd ff d
Explanation:
df fd df f df df dfdf f fdf df df df fd
how to calculate theoretical yield
Answers
Answer:
I'm not really sure how but here's the formula?

To calculate theoretical yield first check chemical equations are balanced. Calculate the mole ratios of the reactants and products, Find the theoretical yield of the reaction.
Percent Yield = Mass of Actual Yield / Mass of Theoretical Yield x 100 percent.
What is theoretical yield ?The theoretical yield is the quantity of product that stoichiometry predicts will be produced, whereas the actual yield is the amount that is actually produced.
The yield of a reaction is used to represent how much of a product is produced from that reaction.
Thus, Divide the ratio by the limiting reactant's molecular weight. The answer is the theoretical yield of the desired product in moles.
To learn more about the theoretical yield, follow the link;
https://brainly.com/question/14966377
#SPJ1
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
Question 1
Describe a procedure you could follow to determine the specific heat of a 45-g piece of metal.
Answers
now that hot metal sample we will put into calorimeter and than wait for it till the water get at its constant stabilizes temperature.
finally we measure temperature of water. we consider no heat around there and now calculate specific heat of the metal by equating the amount of heat lost by the metal to the amount of
Draw a plausible transition state for the bimolecular reaction of nitric oxide with ozone. Use dashed lines to indicate the atoms that are weakly linked together in the transition state.No(g) + o3(g) --> NO2(8) + O2(g)
Answers
One weakly bound intermediate is formed between the oxygen atom of O3 and one of the nitrogen atoms of NO, and another weakly bonded is formed between the oxygen atom of O3 and the nitrogen atom of NO.
Draw a plausible transition state for the bimolecular reaction of nitric oxide with ozone.The bond between nitrogen and oxygen in NO is partially broken, while the bond between the two oxygen atoms in O3 is also partially broken. The bonds between nitrogen and oxygen in NO2 and between the two oxygen atoms in O2 are partially formed.
How do ozone and nitric oxide interact?Nitric oxide and ozone then easily combine to form nitrogen dioxide and oxygen. No net ozone gain occurs as a result of the technique mentioned above. Concentrations are higher in the troposphere than can be explained by these processes alone.
To know more about the bimolecular reaction here;
https://brainly.com/question/1195122
#SPJ1
The United States experienced a decrease in the real GDP, high inflation, and a
rising unemployment rate. The United States
was in the middle of an economic boom
appeared to be entering a recession
was in an economic slump
was in a stagnant economic period
Answers
The United States experienced a decrease in the real GDP, high inflation, and arising unemployment rate.
The United States appeared to be entering a recession.A recession is a decline in economic activity, characterized by declining GDP, high unemployment rates, and increased unemployment benefits. Economic analysts and the media commonly use a two-quarter consecutive decline in real GDP as a definition of a recession.
The United States is considered to have entered a recession in the 1970s, which was characterized by an energy crisis, inflation, and recession. However, by the end of the decade, the economy had improved, and it entered into the 1980s with a strong economic performance.
The 1970s were a period of high inflation, low growth, and an oil crisis, which had a significant impact on the United States economy. Therefore, it can be concluded that The United States was in the middle of an economic boom before the 1970s recession and entered a recession in the 1970s due to a decrease in the real GDP, high inflation, and arising unemployment rate.
For more such questions on inflation visit;
https://brainly.com/question/28061405
#SPJ8
Applying a force can make an electron shift from one atom to another causing what?
Answers
7. What are the coefficients that will balance the skeleton equation below?
N₂ + H₂ → NH3
a.1,1,2
b.3,1,3,1
c.1,1,1,3
d.1,3,3,1
When the equation Fe + Cl₂ → FeCl, is balanced, what is the coefficient
Answers
Answer:
N₂ + 3H₂ → 2NH3
4Fe + 2Cl₂ → 4FeCl
Explanation:
This equations are now balanced
pls rate as brainliest
study this chemical reaction: 2 Zn+ O2= 2 ZnO then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
Answers
The balanced half reaction for the redox reaction of Zn with O2 is,
Oxidation: 2 Zn -------> 2 Zn+2 + 4 e-
Reduction: O2 + 4 e- ---------> 2O-2
What is redox reaction?
Redox reaction includes both oxidation and reduction happening simultaneously where the number of electrons lost in the oxidation is equal to the number of electrons gained in the reduction. There is change in oxidation number of the species involved in redox reaction.
The half cell reaction for redox reaction can be written as,
Zn -------> Zn+2 + 2 e-
Here Zn is undergoing oxidation by losing 2 electrons.
O2 + 4 e- ---------> 2O-2
Here O2 is undergoing reduction by gaining electrons. To make the number of electrons equal the oxidation half cell reaction is multiplied by 2.
Therefore, the redox reaction can be written in half cell reaction as,
Oxidation: 2 Zn -------> 2 Zn+2 + 4 e-
Reduction: O2 + 4 e- ---------> 2O-2
To learn more about redox reaction click on the given link https://brainly.com/question/21851295
#SPJ1
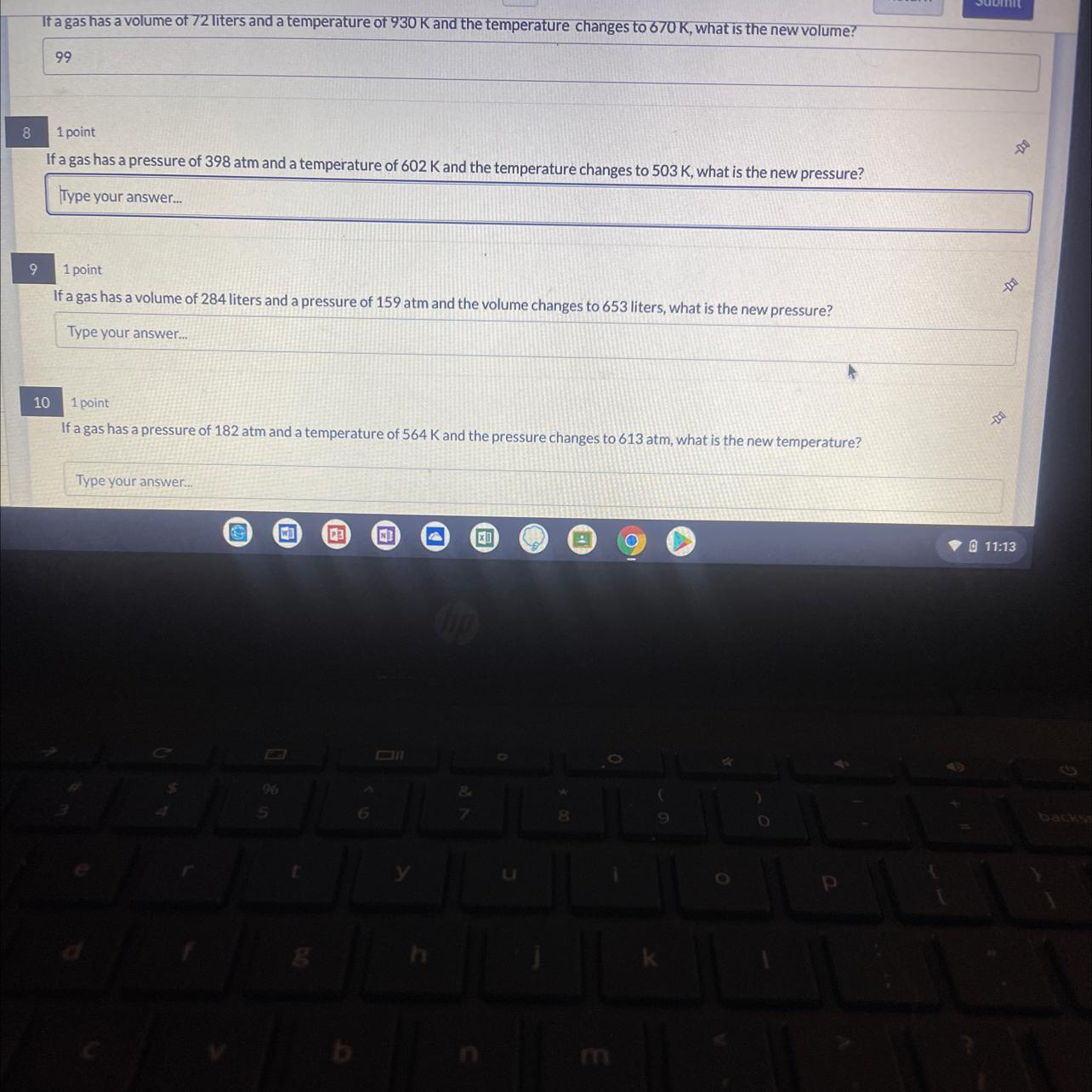
If a gas has pressure of 398 atm and a temp of 602 k and the temp changes to 503 what is the new pressure ?
Answers
answer and explanation
we are given initial temperature and pressure of a gas and a final temperature and we are asked to determine the final pressure
to find this we can use Boyle's law
P1/T1 = P2/T2
we can rearrange to make P2 subject of the formula
P2 = P1T2/T1
= (398 x 602)/503
= 476.3 atm
where is water stored after it has infiltrated deep into the ground?
A. river
B. lake
C. aquifer
D. aquaduct
Answers
Answer:
C. aquifer
Explanation:
I just did a lesson on this
:)
The noble gases are the elements found in group__of the periodic table.
Answers
Answer:
easeaseaseas
Explanation:
Mechanisms (20 points). A suggested mechanism for the reactionNO2( g)+CO(g)⟶CO2( g)+NO(g)is: 1)2NO2( g)⟶NO3( g)+NO(g)2)NO3( g)+CO(g)⟶NO2( g)+CO2( g)Derive the rate law for the production ofCO2(g) . Hint: Assume a steady-state concentration forNO3
Answers
The rate law for the production of \(CO_{2}\)(g) is rate = \(K_{1} [NO_{2}]^{2}\)
The suggested mechanism for the reaction\(NO_{2} (g) + C0(g)\) → \(CO_{2}\)(g) + NO(g) is:
1) \(2NO_{2} (g)\) →\(NO_{3} (g) + N0(g)\)
2) \(NO_{3} (g) + C0(g)\)→\(NO_{2} (g) + CO_{2} (g)\)
To derive the rate law for the production of \(CO_{2} (g)\), we assume a steady-state concentration for \(NO_{3}\). This means that the rate of formation of \\(NO_{3}\) is equal to the rate of its consumption.
For the first step:
rate1 =\(K_{1} [NO_{2}]^{2}\)
For the second step:
rate2 = \(K_{2}\)\(K_{1}\)[\(NO_{3}\)][CO]
Since we assume a steady-state concentration for \(NO_{3}\), rate1 = rate2.
\(K_{1}\)[\(NO_{2}\)]^2 = \(K_{2}\)[\(NO_{3}\)][CO]
Now, we need to solve for [\(NO_{3}\)]:
[NO3] = (\(K_{1}\)/\(K_{2}\))[\(NO_{2}\)\(]^{2}\) / [CO]
The rate law for the production of \(CO_{2}\)(g) is given by the rate of the second step:
rate =\(K_{2}\)[\(NO_{3}\)][CO]
Substitute the expression for [\(NO_{3}\)] from above:
rate = \(K_{2}\)((\(K_{1}\)/\(K_{2}\))[\(NO_{2}\)\(]^{2}\) / [CO])[CO]
Simplify the expression:
rate = \(K_{1}\)[\(NO_{2}\)\(]^{2}\)
So, the rate law for the production of \(CO_{2}\)(g) is:
rate = \(K_{1}\)[\(NO_{2}\)\(]^{2}\)
Know more about steady-state here:
https://brainly.com/question/9128896
#SPJ11
4. Long answer type questions: a. b. C. d. e. f. g. h. j. i. What are the constituent gases of air? Why is the surrounding air not seen with the eyes? How do you prove that air supports burning? How do you show that air occupies space? How do you prove that air has weight? How is air useful to us? Mention any three points. Write any three properties of air. How can you say that air exerts force? Write any four effects of air pollution. Write any three causes of air pollution and any two control measures of it.
Answers
1. The constituent gases of air are:
Nitrogen Oxygen Argon Carbon Dioxide2. The surrounding air is not seen with the eyes because it is transparent. Air molecules are not visible to the na-ked eye, and they do not scatter or absorb visible light significantly. Therefore, air appears colorless and transparent.
What is air?3. To prove that air supports burning, you can perform an experiment with a burning candle. Place a glass jar or bell jar over a lit candle, ensuring that the jar is airtight. As the candle burns, it consumes oxygen from the air inside the jar. Eventually, the candle flame will go out due to the lack of oxygen, proving that air (specifically oxygen) is necessary for burning.
4. To show that air occupies space, you can perform a simple experiment using a plastic bottle or syringe. Fill the bottle or syringe with water, ensuring there are no air bubbles. Then, cover the opening tightly and try to compress the air inside. You will find that it is not possible to compress the air significantly, indicating that air occupies space.
5. To prove that air has weight, you can use a sensitive balance or scale. Weigh an airtight container or balloon, and then fill it with air. The weight of the container or balloon with the added air will be greater than its initial weight, demonstrating that air has weight.
6. Air is useful to us in various ways. Three points highlighting the importance of air are:
Breathing and RespirationCombustion and Energy ProductionClimate Regulation7. Three properties of air include:
Air is Compressible: Air can be compressed or expanded under different conditions, allowing it to fill various spaces and containers.Air has Mass: Air molecules have mass, which means air itself has weight. It exerts pressure on objects and surfaces.Air Exerts Pressure: Due to the collisions of air molecules with surfaces, air exerts pressure in all directions. This pressure is known as atmospheric pressure.Air exerts force in various ways. For example, air pressure allows objects like airplanes to fly by providing lift. Air resistance or drag opposes the motion of objects moving through the air, creating a force that can affect their speed and trajectory.
8. Four effects of air pollution include:
Respiratory ProblemsEnvironmental Damage:Climate ChangeHuman Health Impacts9. Causes of pollution:
Industrial EmissionsVehicle EmissionsResidential and Agricultural Activities10. Two control measures for air pollution include:
Emission ReductionAir Quality RegulationsLearn more about air on https://brainly.com/question/15215203
#SPJ1
Which statement about Fe is supported by the modern atomic theory but not John Dalton’s theory?
A. The element iron is composed of small particles called atoms.
B. The electrons of iron have probable locations in a region of space around the nucleus.
C. Iron atoms combine with other atoms in whole number ratios to form compounds.
D. Chemical reactions that involve iron do not create new atoms of iron.
Answers
Answer:
The correct option is B
Explanation:
One of the claims of John Dalton's atomic theory is that atom is the smallest unit of matter (which suggests that there are no particles smaller than an atom in any matter). This claim has been disproved by the modern atomic theory which established that there are particles smaller than atom (called subatomic particles). These particles are electrons, protons and neutrons.
One of the modern atomic theory was by Neils Bohr, who proposed that electrons move in circular orbits around the central nucleus. Thus, the electrons of iron can also be said to be present in a region of space (circular path) around the nucleus. This proves that option B is the correct option as John Dalton's theory did not even recognize the electron(s) nor the nucleus.
Answer:
b
Explanation:
How many gallons of concentrated sulfuric acid (density of 1.84 g/cm3) are required to have a mass of 184.33 pounds?
Note: 1 kg = 2.204 lb, 1 gallon = 3.79 L.
Answers
After series of conversion from one unit to another, the number of gallons was found to be 12 litres
Density, Mass and Volume
Given Data
Density of Sulfuric acid = 1.84 g/cm3Mass of Sulfuric acid = 184.33 PoundsConversion from pounds to Gram
1 kg ---------2.204 lb
x kg ---------184.33 lb
x = 184.33/2.204
x = 83.634 kg
hence the mass in gram = 83.634*1000
mass in gram = 83634 g
Now let us find the volume
We know that density = mass/volume
volume = mass/density
volume = 83634/1.84
volume = 45453.26 cm^3
Convert cm^3 to litres
Volume in litres = 45453.26 /1000
Volume in litres = 45.45 Litres
Convert from Litres to Gallons
1 gallon = 3.79 L
x gallons = 45.45 L
x = 45.45/3.79
x gallons = 11.99 L
Approx = 12 Litres
Learn more about density here:
https://brainly.com/question/1354972
Why does direct titration of aspirin with NaOH have a side reaction and how to prevent it?
Answers
Direct titration of aspirin with NaOH have a side reaction simply because aspirin is a weak acid
What is direct titration?
Direct titration can simply be defined as a type of titration in which a titrant of known concentration and volume is added to a substance in order to analyze it.
As the name implies, it is called direct titration simply because the one approaches the endpoint of the experiment directly.
Furthermore, the significance of direct titration is that it is used to find the quantity of a substance within a solution with chemical reactions.
So therefore, direct titration of aspirin with NaOH have a side reaction simply because aspirin is a weak acid
Learn more about titration:
https://brainly.com/question/186765
#SPJ1
Which diagram represents a physical change,only?
Answers
Chloroform, CHCl3, has a vapour pressure of 120 mmHg at a certain temperature. Whatis the vapour pressure of a 0,200 m solution of a non-volatile, non-electrolyte solute inchloroform at the same temperature?
Answers
Explanation:
Raoult's law states that the vapor pressure of a solvent that is a over a solution is equal to the vapor pressure of the pure solvent at the same temperature scaled by the mole fraction of the solvent.
Psolution = Xsolvent * P°solvent
Where Psolution is the vapor pressure of the solution, X is the molar fraction of the solvent and P°solvent is the vapor pressure of the pure solvent.
Our solvent is chloroform, we know its vapor pressure.
P°solvent = 120 mmHg
We still have to find the molar fraction of the solvent. The molar fraction of the solvent will be defined like:
Xsolvent = moles of solvent/(total number of moles)
We know that the concentration of the solution is 0.200 m. That means that we have 0.200 moles of solute in each kg of solvent.
In order to find the mole fraction we have to suppose that the solution has 1 kg of solvent.
mass of solvent = 1 kg
molality = 0.200 m
molality = moles of solute/(mass of solvent in kg)
0.200 m = moles of solute/1 kg
moles of solute = 0.200 m * 1 kg
moles of solute = 0.200 moles
So our solution has 0.200 moles of solute in 1 kg of solvent. The solvent is chloroform (CHCl₃). We can convert the mass of solvent into moles using the molar mass of it.
molar mass of CHCl₃ = 119.38 g/mol
mass of solvent = 1 kg = 1 kg * 1000 g/kg
mass of solvent = 1000 g
moles of solvent = 1000 g * 1 moles/(119.38 g)
moles of solvent = 8.377 moles
Our solution has 8.377 moles of solvent and 0.200 moles the non-volatile solute.
total number of moles = moles of solute + moles of solvent
total number of moles = 8.377 moles + 0.200 moles
total number of moles = 8.577 moles
Xsolvent = moles of solvent/(total number of moles)
Xsolvent = 8.377 moles/(8.577 moles)
Xsolvent = 0.977
Finally we found the mole fraction of the solvent and we can find the answer to our problem.
Psolution = Xsolvent * P°solvent
Psolution = 0.977 * 120 mmHg
Psolution = 117.2 mmHg
Answer: The vapor pressure of the solution is 117.2 mmHg.