a.
Neutrons have a neutral charge, a mass of 1 amu, and are found in an atom's ______
b.
Protons are a little smaller than neutrons, they still have a mass of 1 amu though. They are found inside an atom's nucleus and carry a
_______ charge.
C.
Electrons almost have no mass, it's so small we say it is 0 amu. They sail around the nucleus in the _______ They have a ______
charge.
Answers
Neutrons have a neutral charge, a mass of 1 amu, and are found in an atom's Nucleus , Protons are a little smaller than neutrons, they still have a mass of 1 amu though. They are found inside an atom's nucleus and carry a positive charge, Electrons almost have no mass, it's so small we say it is 0 amu. They sail around the nucleus in the orbit, They have a negative charge
Atoms of all elements except for most atoms of hydrogen have neutrons in their nucleus unlike protons and electrons, which are electrically charged, neutrons have no charge they are electrically neutral. that's why the neutrons in the diagram above are labeled n⁰ and the zero stand for zero charge neutron has a neutral charge
Protons are found in the nucleus of the atom and this is a tiny, dense region at the center of the atom protons have a positive electrical charge of one (+1) and a mass of 1 atomic mass unit proton carry positive charge in the nucleus of atom
Electrons are one of three main types of particles that make up atoms and protons and neutrons, which consist of smaller, simpler particles, electrons are fundamental particles that do not consist of smaller particles electron has 0 amu and they have negative charge around the nucleus in the in the orbit called electron
Know more about Neutrons, Protons, Electrons
https://brainly.com/question/28667559
#SPJ1
Related Questions
Which example is NOT a chemical change? *
A. Hydrogen peroxide bubbles in an open wound on your skin.
B. Piece of pizza digested when eaten.
C. Rotting Easter eggs. .
D. Dissolving salt in water.
Answers
Answer:
The answer would be D as no chemical change is occuring.
Explanation:
Answer:
D. Dissolving salt in water.
Explanation:
No chemical change is actually occurring when salt is added to water
True or False
Calorimetry is a general method for measuring heat transfer in substances and chemical reactions.
Answers
how many moles of Fe2O3 will react with 99.0 g of Al
Answers
Taking into account the reaction stoichiometry, 1.83 moles of Fe₂O₃ will react with 99.0 g of Al
Reaction stoichiometryIn first place, the balanced reaction is:
2 Al + Fe₂O₃ → Al₂O₃ + 2 Fe
By reaction stoichiometry, the following amounts of moles of each compound participate in the reaction:
Al: 2 molesFe₂O₃: 1 moleAl₂O₃: 1 moleFe: 2 molesThe molar mass of the compounds is:
Al: 27 g/moleFe₂O₃: 159.7 g/moleAl₂O₃: 102 g/moleFe: 55.85 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al: 2 moles ×27 g/mole= 54 gramsFe₂O₃: 1 mole ×159.7 g/mole= 159.7 gramsAl₂O₃: 1 mole ×102 g/mole= 102 gramsFe: 2 moles ×55.85 g/mole= 111.7 gramsMass of Fe₂O₃ requiredIt is possible to use a simple rule of three as follows: If by reaction stoichiometry 54 grams of Al react with 1 mole of Fe₂O₃, 99 grams of Al react with how many moles of Fe₂O₃?
moles of Fe₂O₃= (99 grams of Al ×1 mole of Fe₂O₃)÷54 grams of Al
moles of Fe₂O₃= 1.83 moles
Finally, 1.83 moles of Fe₂O₃ is needed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
What is the mass of 1 mole of iron atoms?
Answers
Answer:
What is the mass of 1 mole of iron atoms?
Since iron has an atomic mass of 55.847 amu, one mole of iron atoms weighs 55.847 grams.
Explanation:
Got this answer from Angelo State University website.
an element consists of 3 isotopes. isotopes A has an abundance of 45.6 % and it’s mass is 14.0 amu. Isotope B has an abundance of 25.2%, and has a mass of 15 amu, and isotope c has an abundance of 29.2% and it’s mass is 16 amu. What is the atomic mass of the element
Answers
The quantity of protons, neutrons, and electrons that each element has makes it unique.
Each chemical element's atoms has the same number of protons and electrons, which is important because neutrons' quantities are variable.
Isotopes are atoms with the same number of protons but differing numbers of neutrons.
They differ in mass, which affects their physical characteristics even if they have nearly identical chemical properties. There are unstable isotopes that emit radiation as well as stable isotopes that do not. These are referred to as radioisotopes.
Thus, The quantity of protons, neutrons, and electrons that each element has makes it unique.
Learn more about Electrons, refer to the link:
https://brainly.com/question/1255220
#SPJ1
How many grams of lead will be produced if 2.54g of PbS is burned with 1.88g of O2? write the equation
Answers
If 2.54 g of PbS is burned with 1.88 g of O2, approximately 2.20 grams of Pb will be produced.
The balanced equation for the reaction of lead sulfide (PbS) with oxygen (O2) to produce lead (Pb) and sulfur dioxide (SO2) is as follows:
2PbS + 3O2 -> 2Pb + 2SO2
From the balanced equation, we can see that the stoichiometric ratio between PbS and Pb is 2:2 or 1:1. This means that for every 1 mole of PbS, 1 mole of Pb is produced.
To calculate the number of moles of PbS, we need to divide the given mass (2.54 g) by its molar mass:
Molar mass of PbS = 207.2 g/mol (Pb) + 32.07 g/mol (S) = 239.27 g/mol
Moles of PbS = 2.54 g / 239.27 g/mol = 0.0106 mol
Since the stoichiometric ratio between PbS and Pb is 1:1, the number of moles of Pb produced is also 0.0106 mol.
To calculate the mass of Pb, we multiply the number of moles by its molar mass:
Molar mass of Pb = 207.2 g/mol
Mass of Pb = 0.0106 mol x 207.2 g/mol = 2.20 g
This calculation is based on the stoichiometric ratio between PbS and Pb, where 1 mole of PbS produces 1 mole of Pb. By converting the given mass of PbS to moles and then multiplying by the molar mass of Pb, we can determine the mass of Pb produced.
For more such question on PbS. visit :
https://brainly.com/question/27964828
#SPJ8
Father, I come to you worn and weary from the hard times I have walked through recently. I come to you seeking your shelter where I know I can find security and rest in your shadow. Father, you are mighty. I know my circumstances are no match for Your great power. Father I place a healing of these people that they feel a conviction in there heart that they would turn from there wicked way and turn to you father. IN JESUS NAME AMEN
Answers
Answer:
Fact: There’s only one letter that doesn’t appear in any U.S. state name
And that is Q
Explanation:
Can gravity be considered a force? Explain your reasoning
Answers
Answer:
I think that gravity can be considered a force.
Explanation:
As the object falls, it moves faster and faster. Gravity is considered a universal force because it acts between any two masses anywhere in the universe. For example, there is a gravitational pull between the Sun and the Moon. Even small masses attract one another.
Hope this helped! :)
10th grade chemistry. I’m so confused on what I need to do. What do I draw?

Answers
In chemistry, drawing chemical structures is a fundamental skill that allows us to communicate and understand chemical concepts.
A chemical structure is a diagram of a molecule or compound that depicts the arrangement of atoms as well as the chemical bonds that hold them together. Depending on the complexity of the molecule and the level of detail required, there are various methods for drawing chemical structures. Lewis structures, line structures, and condensed structures are the most prevalent types of chemical structures.Lewis structures are diagrams that depict a molecule's valence electrons and bonding patterns. To create a Lewis structure, you must first determine how many valence electrons each atom has and then use them to form bonds and fill octets.
When designing Lewis structures, you should additionally consider formal charges, resonance, and electronegativity.Line structures are a method of depicting molecules that is simplified by the use of lines to represent bonds and atoms. In line structures, each vertex or endpoint represents a carbon atom unless otherwise indicated. Hydrogen atoms are not usually shown unless they are attached to a heteroatom. Line structures are useful for quickly drawing and comparing structures, but they do not show the three-dimensional arrangement of atoms.Condensed structures are another way of representing molecules by using symbols and abbreviations to represent atoms and functional groups. In condensed structures, bonds are implied rather than drawn, and atoms are listed in the order of their connectivity.
Condensed structures are useful for representing large and complex molecules, but they can be ambiguous and difficult to read without practice.
Overall, drawing chemical structures is an essential skill in chemistry that requires practice and attention to detail. By learning how to draw Lewis structures, line structures, and condensed structures, you can better understand chemical concepts and communicate your ideas to others.
for such more questions on chemical
https://brainly.com/question/29886197
#SPJ8
Look at the structure of ethane below and answer the following questions:
A. Calculate the electronegativity difference between the C and H atoms using the table below.
B. Where would the partial + and - changes be?
C. Is the ethane molecule more or less polar than water? Why or why not?
D. If the oceans were filled with ethane rather than water how might they be different? (Hint: think about hydrogen bonding)

Answers
The Electronegativity Difference between the C and H atoms in ethane would be 0.35.
The structure of ethane is as follows:A) Electronegativity of Carbon (C) is 2.55, and Hydrogen (H) is 2.20. Electronegativity Difference (ΔEN) = 2.55 - 2.20 = 0.35B) There would be a partial negative charge on the Carbon atom, and there would be a partial positive charge on the Hydrogen atoms.C) The ethane molecule is less polar than water. This is because the electronegativity difference between the Carbon and Hydrogen atoms is low (0.35) in ethane, whereas the difference between the Oxygen and Hydrogen atoms is high (1.4) in water. Due to the high electronegativity difference in water, it creates a dipole moment, making it a polar molecule. Whereas ethane has no dipole moment and is considered a nonpolar molecule.D) If the oceans were filled with ethane instead of water, then there would be no hydrogen bonding. As a result, many of the physical properties of the ocean would be different.
For example, the freezing point of the ocean would be much lower because of the weak intermolecular forces between ethane molecules. Due to the absence of hydrogen bonding, the viscosity of the oceans would be less than that of water, leading to easier movement of organisms.
for such more questions on Electronegativity
https://brainly.com/question/24977425
#SPJ8
According to Le Chatelier's Principle, adding reactants to a chemical reaction at equilibrium will cause the reaction to

Answers
The correct option is B as The reaction proceeds to the right, increasing the formation of products.
What is Le Chatelier's Principle?Le Chatelier’s principle states that if a system in a state of dynamic equilibrium is disturbed by a change to its conditions, then the position of equilibrium will shift to counteract the change. The addition of a catalyst, actually, has no impact on the position of equilibrium.
This is because catalysts speed up the rates of both the forward and reverse reactions. This means that the two rates remain equal, as they both change by the same amount. Adding more reactants to a chemical reaction shifts the equilibrium in the direction of the products; therefore, the equilibrium shifts to the right.
Learn more about Le Chatelier's Principle:
https://brainly.com/question/9694955
#SPJ1
Predict and explain the structure of the major and minor products when hydrogen bromide is added to 2-methylbut-2- ene, (Ch3)2CCHCH3
Pls help with homework!!!!
Answers
When hydrogen bromide (HBr) is added to 2-methylbut-2-ene ((CH3)2CCHCH3), an electrophilic addition reaction takes place, where the π bond of the alkene is broken, and the hydrogen and bromine atoms are added to the resulting carbocation.
The reaction proceeds through a Markovnikov addition, where the hydrogen atom attaches to the carbon atom with the greater number of hydrogen atoms.
In this case, the initial addition of HBr to 2-methylbut-2-ene leads to the formation of a primary carbocation, as the positively charged carbon atom only has one alkyl group attached to it. The primary carbocation is relatively unstable, and it can undergo a rearrangement to form a more stable secondary carbocation.
The major product that is typically obtained is the 2-bromo-2-methylbutane. The hydrogen atom from HBr adds to the carbon with three hydrogen atoms (the more substituted carbon), resulting in the formation of a secondary carbocation.
On the other hand, a minor product is also formed, which is 3-bromo-2-methylbutane. This product arises from the addition of HBr to the primary carbocation, which is less stable. Although the primary carbocation is less favored, it can still be formed and lead to the formation of the minor product.
In summary, the addition of HBr to 2-methylbut-2-ene yields two products: the major product is 2-bromo-2-methylbutane, resulting from the addition of HBr to the more stable secondary carbocation, and the minor product is 3-bromo-2-methylbutane, originating from the less stable primary carbocation.
For more such questions on electrophilic addition visit:
https://brainly.com/question/9643304
#SPJ8
HELP ME PLEASE I BEG YOU ITS DUE IN 2 HOURS !! ASAP !!

Answers
Answer:
1) Element is a pure substance consisting only of atoms that all have the same numbers of protons in their nuclei.
2) a) Element is a pure substance
b) It is homogeneous
3) Bromine
4) Elements and compounds are pure chemical substances found in nature. The difference between an element and a compound is that an element is a substance made of same type of atoms, whereas a compound is made of different elements in definite proportions. Examples of elements include iron, copper, hydrogen and oxygen.
5) The law of definite proportions
Explanation:
This is all i know, bro
1. What is the product of sodium metal reacting with chlorine gas?
Answers
Answer:
That would be sodium chloride! I hope this helps! :)
Solve out like the boxes are shown like

Answers
Final enthalpy for 0.600 moles of H₂O(g) is given as ΔH = -407 kJ
Given reaction is as follow,
BH₃(g) + 3O₂(g) → B₂H₂(g) + 3H₂O(g)
Now,
We need to find final enthalpy change (ΔH) for 0.600 moles of H₂O(g).
Again,
We know that change in enthalpy is an extensive property or in other words, ΔH is directly proportional to the moles of reactant.
For 3 moles of H₂O(g) , ΔH= -2035kJ
For 0.600 moles of H₂O(g) , ΔH = -2035 × 0.600/3
ΔH = -407 kJ
Thus from the above conclusion we can say that , final enthalpy for 0.600 moles of H₂O(g) is given as ΔH = -407 kJ
Learn more about Enthalpy here: https://brainly.com/question/27207707
#SPJ9
definition of formula in science. (own words)
Answers
A hit-and-run accident has left a pedestrian injured. The investigators have used eyewitness testimony and motor vehicle records to narrow down the list of suspects. When one of these suspects is interviewed, he is asked if they would find any blood on his car. He says that he hit a deer a few weeks ago, so it is possible that there is blood. How could this statement be tested?
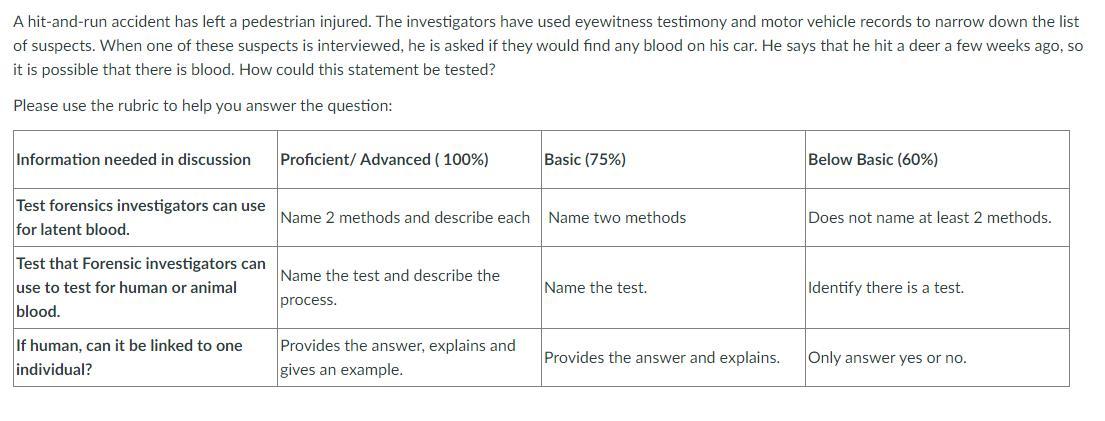
Answers
Test forensic investigators can use to test for human or animal blood will be
required to test the statement.
What is Forensics?These are different tests and techniques used by appropriate body to
investigate a crime in order to know what really happened and who is
responsible.
The test which can be used to differentiate between human and animal
blood is Precipitin Test. This is done by identifying the presence of proteins
that are found only in human blood such as albumin.
Read more about Blood tests here https://brainly.com/question/3731326
What sample at STP has the same number of molecules as 5 L of NO2
Answers
Answer:
5l NO
2
at STP
No. of molecules=
22.4
5
mol=
22.4
5
×N
A
molecules
A) 5ℊ of H
2
(g)
No. of moles=
2
5
mol=
2
5
×N
A
molecules
B) 5l of CH
4
(g)
No. of moles of CH
4
=
22.4
5
mol=
22.4
5
N
A
molecules
C) 5 mol of O
2
=5N
A
O
2
molecules
D) 5×10
23
molecules of CO
2
(g)
Molecules of 5l NO
2
(g) at STP=5l of CH
4
(g) molecules at STP
Therefore, option B is correct.
Was this answer helpful?
what is the oxidation state of nitrogen in a nh4f molecule
Answers
Answer:
+3
Explanation:
NH₄F
=> N + 4H + F = 0
=> N + 4(+1) + 1(-7) = 0
=> N = 7 - 4 = +3
what does the word "local" mean
Answers
Answer:
belonging or relating to a particular area or neighborhood, typically exclusively so
Explanation:
According to the Law of Conservation of Matter, if I have an Oreo cookie that weighs 20 grams and I crush it into crumbs for a recipe, how much do the crumbs weigh before I add anything else to them?
Answers
The Law of Conservation of Matter states that matter in any form cannot be created nor destroyed. With this being said, an Oreo cookie can be crushed into crumbs and still weigh the same because no matter is destroyed in the process.
Unit Test Unit Test Active 13 TIME REMAINING 01:31:26 Chemical A and Chemical B react in an exothermic reaction. What can be known about what will happen when Chemical A and Chemical B are mixed together? O The new substance will need more energy to form its chemical bonds than the old substance will release. More energy will be released from the old substance than the new substance will need to form its chemical bonds. The color will change as a result of the reaction. O The substance will bubble as a result of the reaction. Save and Exit Next Submit Mark this and return
Answers
Answer:
More energy is released from the old substance than the new substance needs to form its chemical bonds
Explanation:
Chemical A and Chemical B react in an exothermic reaction. When Chemical A and Chemical B are mixed together more energy will be released from the old substance than the new substance will need to form its chemical bonds. Therefore, option B is correct.
What is an exothermic reaction ?When a chemical reaction goes on, energy is shifted to or from the surroundings. When energy is moved to the surroundings, this is called an exothermic reaction, and the temperature of the surroundings increases.
Examples of exothermic reactions are combustion reactions and many oxidation reactions.
A chemical reaction is exothermic if heat is passed by the system into the surroundings. Because the surroundings are deriving heat from the system, the temperature of the surroundings increases.
Thus, option B is correct.
To learn more about the exothermic reaction, follow the link;
https://brainly.com/question/10373907
#SPJ6
4.Calculate the Hydroxide, Hydrogen ion and POH of solution if the PH of solution is 7.b
5.Solution A Has PH =4 and solution B has PH = 7.How many times greater is the Hydroxide ion
concentration in solution A than the Hydronium ion concentration in solution B
Answers
the ph is gonna be your value and the 4 is gonna be your main subject
so as the ph is your value u gonna ad your ph and 7 and 4 toghter then multiple your answer 2 times because ph represent multiple and your value
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
teacher
24. When snow or ice
(considered the
solid form of water) changes
directly into vapor (dry ice).
EUX
Answers
Answer:
Solid form of water is solidification
You may have noticed that all the elements in the first column of the periodic table, the alkali metals, have a 1 charge when they combine with negative ions. Another group of positive ions are the alkaline earth metals located in the second column of the periodic table. What charge is typical for ions of the alkaline earth metals?
Answers
Answer:
The charge that is typical for ions of the alkaline earth metals is +2
Explanation:
Group 1 elements (alkali metals such as Li, Na, K...) all have one valence electron that they donate when forming ionic bonds. This will cause them to have a charge of +1.T he alkaline earth metals (Ca, Mg) have two valence electrons they donate which means their charge when forming ions is +2. The members of the aluminum family lose three electrons giving them a charge of +3.
How are gluons involved in forces within the atom?
A. Electrostatic forces use gluons to hold electrons together .
B. Gluons cause protons to repel each other in the nucleus.
C. Gluons are emitted as radioactive particles by the weak nuclear force .
D. Strong nuclear forces act through gluons in the nucleus.
Answers
Answer:
D
Explanation:
Took the test!
Gluons act as exchange particles and are known as gauge bosons. They are involved in the forces within the atom as a strong nuclear force that binds the nucleus. Thus, option D is correct.
What is a strong nuclear force?A strong nuclear force is a type of interaction that is present inside the atom and involves the nucleus. They bind the quarks to make the neutron and the proton, sub-atomic particles.
The strong nuclear force holds the atomic nucleus together and depends on the spin but not the charge. These forces are due to the interaction between the gluons and are stronger than the chemical bonds. The exchange of the mesons results in nucleons that lead to a strong nuclear force.
Therefore, option D. the gluons are responsible for the strong nuclear forces that bind the nucleus.
Learn more about strong nuclear forces here:
https://brainly.com/question/12653804
#SPJ5
if two magnets are placed on a table, which statement describes a situation with the most attraction between the two magnets
Answers
The north pole of one magnet is near the South pole of the other magnet.
The ends of a magnet are called its poles. One end is called the north pole, the other is called the south pole. If you line up two magnets so that the south pole of one faces the north pole of the other, the magnets will pull toward each other.
Calculate the mass (in kg) of 4.87 x 10 25 atoms of Zn
Answers
Answer:
5.29kg
Explanation:
5.29kg
The mass of 4.87×10²⁵ atoms of Zn is 5.29 Kg
From Avogadro's hypothesis,
6.02×10²³ atoms = 1 mole of ZnRecall,
1 mole of Zn = 65.38 g
Converting 65.38 g to Kilogram (Kg), we have:
1000 g = 1 Kg
Therefore,
65.38 g = 65.38 / 1000
65.38 g = 0.06538 KgThus, we can say that,
6.02×10²³ atoms = 0.06538 Kg of ZnFinally, we shall determine the mass of Zn that contains 4.87×10²⁵ atoms. This can be obtained as follow:
6.02×10²³ atoms = 0.06538 Kg of Zn
4.87×10²⁵ atoms = \(\frac{4.87*10^{25} * 0.06538}{6.02*10^{23} } \\\\\)
4.87×10²⁵ atoms = 5.29 Kg of ZnTherefore, the mass of 4.87×10²⁵ atoms of Zn is 5.29 Kg
Learn more: https://brainly.com/question/5809151
Which of the images shows a phase of matter where there is no specific shape or volume?
