Answers
From the titration curve that have been shown in the image, the equivalence point is 50 mL
What is the equivalence point on a titration curve?At the equivalence point on a titration curve, the amount of titrant added is chemically equivalent to the amount of analyte in the sample being evaluated. As a result of the reaction between the titrant and analyte at this point, the entire analyte has been neutralized by the titrant.
You can locate the equivalence point by plotting the pH or any relevant aspect of the sample under examination as a function of the volume of titrant used.
Learn more about a titration curve:https://brainly.com/question/30826030
#SPJ1
Related Questions
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
Which of the following is the least important property of a mineral?
A streak
B hardness
C luster
D color
Answers
Explanation:
Türküm lben .Türk varmi kardaşlar!!!
Answer:
D color
Explanation:
One physical test for minerals is to check their color. Certain kinds of minerals always have a similar color, but most minerals have a range of colors. This is the least informative property of a mineral. Some words to describe color are: pale, bright, streaked, splotchy, banded, and speckled.
This is from my school assignment good luck everyone!! :D
the mechanism of a reaction 2-nitropentane + NaOH/H3O+
Answers
The interaction of sodium hydroxide (NaOH), hydronium ion (H₃O⁺), and 2-nitropentane. It appears to involve both acid-base reactions and nucleophilic substitution. Here is a theory for the reaction's mechanism:
Step 1: Deprotonation
Strong base NaOH sodium hydroxideinteracts with hydrogen ion H₃O⁺ to produce water (H₂O) and sodium hydronium ion (NaH₃O⁺):
H₃O⁺ + NaOH → H₂O + NaH₃O⁺
Step 2: Nucleophilic Attack
The carbon-nitrogen double bond in NaH₃O⁺ is attacked by the deprotonated nitropentane anion, which is produced from 2-nitropentane, acting as a nucleophile:
NaH₃O⁺ + Nitropentane → Na+ + Nitropentane Anion
Step 3: Protonation
The end product, 2-nitropentanol, is created when water (H₂O), acting as a proton donor, donates a proton to the nitropentane anion:
Nitropentane Anion + H₂O → 2-Nitropentanol
The complete reaction can be summarized as follows:
2-nitropentane + NaOH/H₃O⁺ → 2-nitropentanol + Na⁺ + H₂O
Learn more about sodium hydroxide, here:
https://brainly.com/question/29092241
#SPJ1
Hybridization of Al in AlF3
Answers
Answer:
Hybridization of Al in AlF3 solid is sp3d2.
What effects does human population have on forest trees
Answers
Explanation:
Depreciation on fixed assets is recorded in the accounts of:
general fund D. capital projects fund
enterprise fund E. debt service fund
special Revenue fund
hexaphosphorus nonasulfide formula
Answers
Answer:
P6S9
Explanation:
Firstly, let's write the numbers in Latin
1 = mono
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
Secondly, write the symboles of the given elements:
Phosphorus is P
Sulfide is S
Finally, connect the numbers and symbols.
Rule of pronunciation: Number of first element + symbol of first element + number of second element + symbol of second element
P6S9
Please upvote.
How many grams of HF are needed for 182 grams of SiO2 to react completely? Express your answer to three significant figures.

Answers
1) Check if the chemical equation is balanced.
\(\text{SiO}_2+4HF\rightarrow SiF_4+2H_2O\)2) Convert grams of SiO2 into moles
\(molesofSiO_2=182gSiO_2\cdot\frac{1moleofSiO_2}{60.0843gSiO_2}=3.0291\)3) How many moles of HF reacts
\(\text{molesofHF}=3.0291molesofSiO_2\cdot\frac{4\text{molesofHF}}{1moleofSiO_2}=12.1163\)4) Convert moles of HF into grams
\(\text{gramsofHF}=12.1163\text{molesofHF}\cdot\frac{20.0063\text{gof HF}}{1\text{moleofHF}}=242.4025\text{ g of HF}\)The reaction requires 242 grams of HF
two uses of sodium carbonate
Answers
Sodium carbonate, also known as washing soda or soda ash, has a wide range of applications. Sodium carbonate can be naturally occurring or synthetically produced through various methods, including the Solvay process, which is the most common method of industrial production.
Sodium carbonate, also known as washing soda or soda ash, has many uses, including:
1) Cleaning agent: Sodium carbonate is an effective cleaning agent due to its alkaline nature. It is used in laundry detergents and household cleaners to remove stains and grease from clothes and surfaces.
2) Industrial applications: Sodium carbonate is used in a variety of industrial applications. It is used in the production of glass, pulp and paper, and soaps and detergents. It is also used as a water softener and pH regulator in chemical processes.
Learn more about Sodium Carbonate at
brainly.com/question/31344166
#SPJ1
When initially set up, in which direction does the thermal energy between the flasks flow?
A
Thermal energy flows from the flask on the left to the flask on the right.
B
Thermal energy flows from the flask on the right to the flask on the left.
C
Thermal energy does not flow between the two flasks.
D
Thermal energy flows equally between the two flasks.

Answers
Answer:
How you going to delete my answer even though it was right but dont delete his answer bro didnt even say nun
Explanation:
Answer D
Thermal energy flows from the flask on the left to the flask on the right as energy is transferred from higher to lower temperature.
What is thermal energy?
Thermal energy is defined as a type of energy which is contained within a system which is responsible for temperature rise.Heat is a type of thermal energy.It is concerned with the first law of thermodynamics.
Thermal energy arises from friction and drag.It includes the internal energy or enthalpy of a body of matter and radiation.It is related to internal energy and heat .It arises when a substance whose molecules or atoms are vibrating faster.
These vibrating molecules and atoms collide and as a result of which heat is generated in a substance , more the collision of particles , higher is the thermal energy.
Learn more about thermal energy,here:
https://brainly.com/question/3022807
#SPJ2
if one gram of sulphur dioxide contains x molecules what will be the number of molecules in 1g of methane
Answers
The ratio of molecules in sulphur dioxide and methane will be the same as the ratio of their moles. So, first of all we should find out the number of moles of sulphur dioxide in 1 gram of sulphur dioxide in 1 gram of sulphur dioxide, and the number of moles of methane in 1 gram of methane. This can be done as follows :
(i) The molecular formula of sulphur dioxide is \(SO_{2}\)
So, \(1\) mole of \(SO_{2}\) = \(Mass\) \(of\) \(2'O'\)
\(=32+2*16\)
\(= 64\) grams
Now, \(64g\) of sulphur dioxide \(= 1\) mole
So, \(1g\) of sulphur dioxide = \(\frac{1}{64}\) mole
Thus, we have \(\frac{1}{64}\) mole of sulphur dioxide and it contains molecules in it. Now, since equal moles of all the substance contain equal number of molecules, therefore, \(\frac{1}{64}\) mole of methane will also contain x molecules of methane.
(ii) Molecular formula of methan is \(CH_{4}\)
So, 1 mole of \(CH_{4}\) = Mass of C + Mass of 4 H
\(=12+4*12\)
Now, 16g of methane = 1 mole
So, 1 g of mathane = \(\frac{1}{16}\) mole
We know that:
\(\frac{1}{64}\) mole of methane contains = x molecules
So, \(\frac{1}{16}\) mole of contains will contain =\(\frac{x*64}{16}\) molecules
=\(4x\) molecules
How many moles of NaHCO3
are in 27.5 g NaHCO3
?
Answers
Using dimensional analysis write the dimension of pressure with its unit
Answers
The unit commonly used to measure pressure is the Pascal (Pa), which is equivalent to a force of 1 Newton per square meter: [Pressure] = 1 [kg/s²·m] = 1 Pa
Pressure is defined as the force per unit area. It can be represented using dimensional analysis by considering the fundamental units of force and area. Force is represented by the fundamental unit of mass (kg) multiplied by the fundamental unit of acceleration (m/s²). Therefore, the dimension of force is [kg·m/s²]. Area is represented by the fundamental unit of length (m) squared. Therefore, the dimension of area is [m²]. To determine the dimension of pressure, we divide the dimension of force by the dimension of area: [Pressure] = [Force] / [Area] = [kg·m/s²] / [m²]
Simplifying the expression, we can cancel out the common unit of length:
[Pressure] = [kg/s²·m] The unit commonly used to measure pressure is the Pascal (Pa), which is equivalent to a force of 1 Newton per square meter: [Pressure] = 1 [kg/s²·m] = 1 Pa Therefore, the dimension of pressure is [kg/s²·m] and its unit is the Pascal (Pa).
For more question on pressure
https://brainly.com/question/24719118
#SPJ8
An object submerged into 50 cm 3 of water raises the water level to 65 cm 3. The mass of the object is 30 g. What is its density?
450 g/cm^3
45 g/cm3
15 gam3
2 glam
Answers
The density of the object is 2 g/cm³
Density of an object is defined as the mass of the object per unit volume of the object i.e
Density = mass / volumeTo obtain the answer to the question given above, we'll begin by calculating the volume of the object. This can be obtained as follow:
Volume of water = 50 cm³
Volume of water + Object = 65 cm³
Volume of object =? Volume of object = (Volume of water + Object) – (Volume of water)Volume of object = 65 – 50
Volume of object = 15 cm³Finally, we shall determine the density of the object. This can be obtained as follow:
Volume of object = 15 cm³
Mass of object = 30 g
Density of object =?Density = mass / volume
Density = 30 / 15
Density of object = 2 g/cm³Therefore, the density of the object is 2 g/cm³
Learn more: https://brainly.com/question/24563659
Hey to all beautiful people behind the screen. If you're kindhearted and have some spare time, i would like you to help me with one simple question of mine.
For example, 2KOH. It means that there is 2 mole of KOH right?(correct me if I'm wrong) So can i say that there is 2 mole of K ions and 2 mole of OH ions?
Sorry if this question sounds idiotic but I'm just confused rn
Please help and enlighten me, people. Your help will be very much appreciated
Answers
Answer:
Yes that is 2 moles of KOH.
Before the reaction K is K+1 and OH is OH-1 now when they reacts they interchange and cancel so it becomes neutral and not in an ionic state
which of the following is included in some surface disinfectants as the actual disinfecting chemical? sodium lauryl sulfate formalin phenol bichloride of mercury
Answers
The actual disinfecting chemical which is included in some surface disinfectants is Phenol.
A chemical agent or compound known as a disinfectant is used to inactivate or eradicate bacteria on inert surfaces.
Alcohols and aldehydes are the chemical sterilant and high-level disinfectants. To eliminate the microorganisms that are found in drains, toilets, and floors, disinfectants like phenol are utilized.
In addition to alcohol, The EPA and the Centers for Disease Control have approved a class of effective surface sanitizers and disinfectants based on quaternary ammonium cations for use as hospital-grade disinfectants.
Aldehydes, like formaldehyde and glutaraldehyde, are sporicidal, and fungicidal, and have a broad range of microbicidal activity. They have a little amount of residual activity and are partially inactivated by organic materials.
To know more about, disinfectants :
brainly.com/question/28486089
#SPJ4
Helppppp pleaseeee xxxxxx
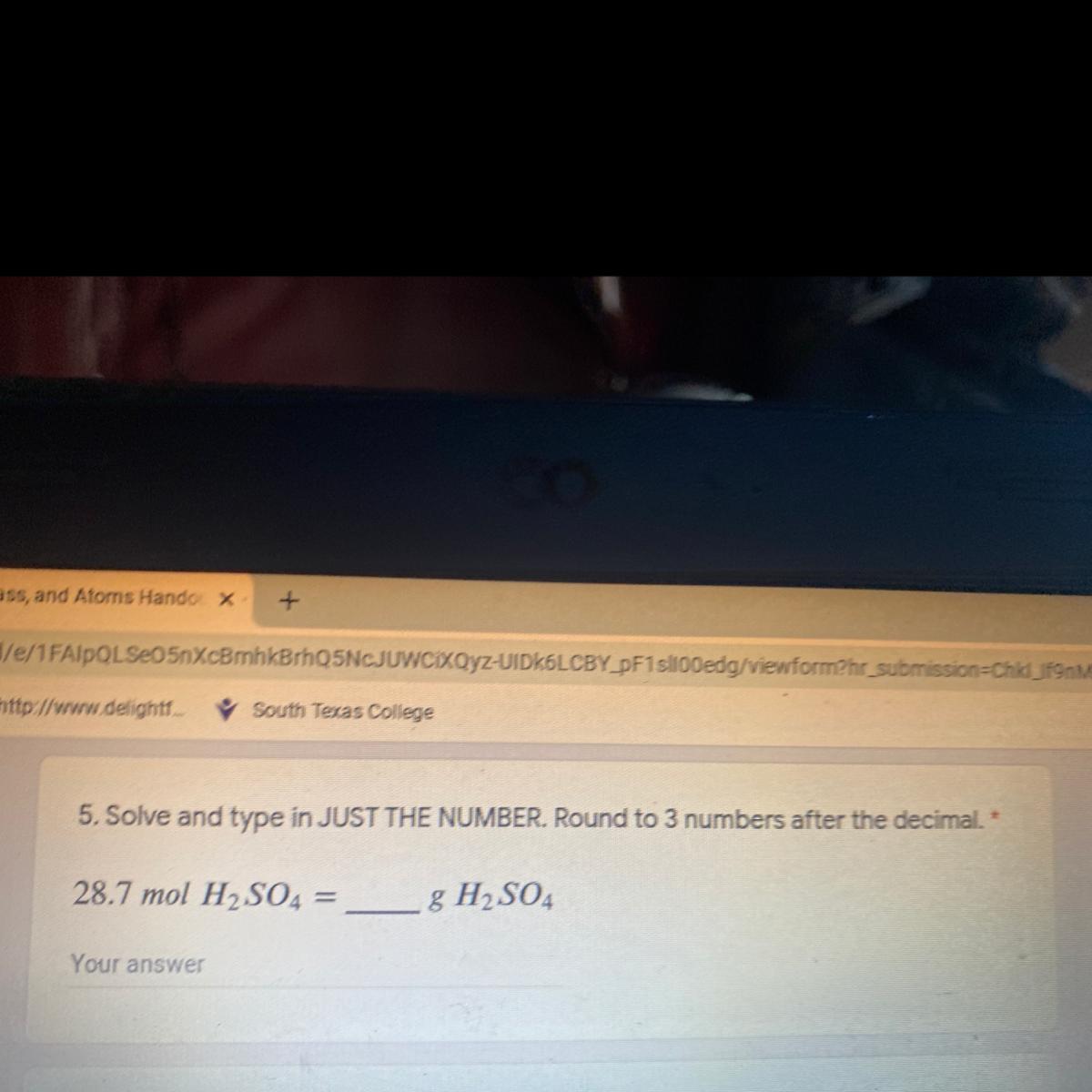
Answers
Answer:
2812.6 g of H₂SO₄
Explanation:
From the question given above, the following data were obtained:
Mole of H₂SO₄ = 28.7 moles
Mass of H₂SO₄ =?
Next, we shall determine the molar mass of H₂SO₄. This can be obtained as follow:
Molar mass of H₂SO₄ = (1×2) + 32 + (16×4)
= 2 + 32 + 64
= 98 g/mol
Finally, we shall determine the mass of H₂SO₄. This can be obtained as follow:
Mole of H₂SO₄ = 28.7 moles
Molar mass of H₂SO₄ =
Mass of H₂SO₄ =?
Mole = mass / Molar mass
28.7 = Mass of H₂SO₄ / 98
Cross multiply
Mass of H₂SO₄ = 28.7 × 98
Mass of H₂SO₄ = 2812.6 g
Thus, 28.7 mole of H₂SO₄ is equivalent to 2812.6 g of H₂SO₄
Arrange these species into isoelectronic groups. It does not matter which group goes in which box, so long as the correct species are grouped. Isoelectronic group A Isoelectronic group B Isoelectronic group C Answer Bank
Answers
The species to be arranged are;
Sr2+, N3-, Li+, Ne, Br-, B3+, Al3+, He, Y3+
Answer:
Group A (10 electron species)
Al^3+, Ne, N^3-
Group B (36 electron species)
Sr^2+, Y^3+, Br^-
Group C ( 2 electron species)
He, Li^+, B^3+
Explanation:
Atoms and ions that have the same electron configuration are said to be isoelectronic. The species may not belong to the same group in the periodic table but are connected by the fact that they all have the same number of electrons. Cations and anions may belong to the same group of isoelectronic species provided that they all have the same number of electrons and the same electronic configuration.
Hence, in each group of isoelectronic species, one electronic configuration can be written for all the species and it will accurately represent the number of electrons for all species in the group since they have the same number of electrons.
For instance, all group C members have the electronic configuration, 1s2. This means that they all possess only two electrons.
You are eating a pizza. What type of mixture are you consuming?
Answers
Answer:
heterogeneous mixture
Explanation:
because pizza has different phases I think
The type of mixture that pizza can be classified is Heterogeneous mixture
Heterogeneous mixture can be regarded as a type of mixture that has it's composition not uniform throughout the entire mixture.Heterogeneous mixture is usually comprised of two or more , instance of this is combination of oil and water.A pizza can be considered as heterogeneous mixture, this is because it doesn't appear as non-uniform substance, it's composition the same at all point in the mixture.The components that made of pizza can be separated by use of physical means.Therefore, heterogeneous mixture can be regarded as one having more than a substance.
Learn more at : https://brainly.com/question/5139963?referrer=searchResults
Basilosaurus cetoides was a large mammal that existed 35 million years ago. It was 20 m long and had a large skull, incompletely formed legs, short arms, and a long tail. Scientists claim that the blue whale, which first appeared 1.5 million years ago, is related to Basilosaurus cetoides. Which argument based on the evidence helps support the scientists’ claim?
Answers
Answer: hey so I’m taking the same test as you right now. I’m on that question and I searched it up and came on here. Would you mind telling me the answer?
Explanation:
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
100 POINTS!!!
What is the average rate of the reaction over the entire course of the reaction?
1.6 × 10−3 (?)
1.9 × 10−3 (?)
2.0 × 10−3 (X)
2.2 × 10−3 (X)

Answers
Answer:
b. 1.9 × 10-3
Explanation:
Answer:1.9x10-3
Explanation:
average
What is the density of an unknown compound in g/ml if 1.28 pounds of the compound has a volume of 4.50L
Answers
1.28 pounds * 453.59 grams/pound = 580.61 grams
Next, we can use the formula for density:
Density = Mass / Volume
Density = 580.61 grams / 4.50 L
Density = 128.91 g/L
Therefore, the density of the unknown compound is 128.91 g/L or 0.12891 g/mL (since there are 1000 mL in 1 L).
How many moles are in 3.01x10^23 atoms of zinc?
Answers
Answer:
Therefore the atomic mass on the periodic table is also the mass of 1 mole of those atoms in grams. 6. 1. H. 1.0079. 1 mol. 23. 6.02 x 10 ...
Explanation:
Pls follow me
There are 0.500 moles in \(3.01\times 10^{23}\) atoms of zinc.
Explanation:
Given:
The \(3.01\times 10^{23}\) atoms of zinc.
To find:
The moles of zinc
Solution:
The number of zinc atoms =\(N= 3.01\times 10^{23}\)
Moles of zinc = n
According to the mole concept;
\(1 mole = N_A=6.022\times 10^{23} atoms/ molecules/ions\)
In n moles of zinc, there are N atoms of zinc.
\(N=n\times N_A\)
\(3.01\times 10^{23} =n\times6.022\times 10^{23}\\n=\frac{3.01\times 10^{23} }{6.022\times 10^{23}}\\=0.500\)
There are 0.500 moles in \(3.01\times 10^{23}\) atoms of zinc.
Learn more about the mole concept here:
brainly.com/question/24198197
brainly.com/question/7287712?referrer=searchResults
Limiting reactant question!

Answers
The limiting reactant is N₂O₄ and the mass of N₂ formed from the reaction is 45.7 g (Option C)
How do I determine the limiting reactant?The limiting reactant can be obtained as illusrated below:
N₂O₄ + 2N₂H₄ -> 3N₂ + 4H₂O
Molar mass of N₂O₄ = 92.02 g/molMass of N₂O₄ from the balanced equation = 1 × 92.02 = 92.02 gMolar mass of N₂H₄ = 32.05 gMass of N₂H₄ from the balanced equation = 2 × 32.05 = 64.1 g Molar mass of N₂ = 28.02 gMass of N₂ from the balanced equation = 3 × 28.02 = 84.06 gFrom the balanced equation above,
92.02 g of N₂O₄ reacted with 64.1 g of N₂H₄
Therefore,
50 g of N₂O₄ will react with = (50 × 64.1) / 92.02 = 34.83 g of N₂H₄
From the above calculation, we can see that only 34.83 g of N₂H₄ out of 45.0 g reacted.
Thus, the limiting reactant is N₂O₄
How do I determine the mass of N₂ formed?The limiting reactant shall be used in this case in order to obtain a maximum yield of N₂. Details below:
92.02 g of N₂O₄ reacted to produce 84.06 g of N₂
Therefore,
50 g of N₂O₄ will react to produce = (50 × 84.06) / 92.02 = 45.7 g of N₂
Thus, the mass of N₂ formed is 45.7 g
Therefore, the correct answer to the question is (Option C)
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
Which statement is correct about the properties that define a particular kind of mineral?(1 point) Responses A particular mineral is defined by neither its chemical composition nor crystalline structure. A particular mineral is defined by neither its chemical composition nor crystalline structure. A particular mineral is defined only by its crystalline structure. A particular mineral is defined only by its crystalline structure. A particular mineral is defined only by its chemical composition. A particular mineral is defined only by its chemical composition. A particular mineral is defined by its chemical composition and crystalline structure.
Answers
Answer:A particular mineral is defined by its chemical composition and crystalline structure.
Explanation:
Statement which is true about minerals is that a particular mineral is defined by its chemical composition and crystalline structure.
What are minerals?Minerals are defined as a chemical compound which has a well -defined composition and possesses a specific crystal structure.It occurs naturally in the pure form.
If a compound occurs naturally in different crystal structure then each structure is considered as a different mineral.The chemical composition of a mineral varies depending on the presence of small impurities which are present in small quantities.
Some minerals can have variable proportions of two or more chemical elements which occupy equivalent position in the crystal structure.It may also have variable composition which is split into separate species.
Physical properties of minerals include color,streak, luster,specific gravity and cleavage.
Learn more about minerals,here:
https://brainly.com/question/18078524
#SPJ2
How much will this EROSION change the elevation at location W, to the nearest meter?

Answers
Answer:
1,329
Explanation:
under which of the following conditions of temperature and pressure will h2 gas be expected to behave most like an ideal gas? responses 50 k and 0.10 atm 50 k and 0.10 atm 50 k and 5.0 atm 50 k and 5.0 atm 500 k and 0.10 atm 500 k and 0.10 atm 500 k and 50 atm
Answers
H₂ gas expected to behave most like an ideal gas in condition of temperature 500 K and pressure 0.1 atm.
When temperatures and pressures are extremely high and low, real gases will behave like ideal gases. Nevertheless, actual gases are less likely to behave optimally at lower temperatures and pressures.
PV=nRT is the ideal gas equation, where P is the gas pressure.
V = Gas Volume
Gas constant: R
T = gas temperature
n = the quantity of gas moles.
When the amount of gas molecules and the intermolecular interactions between them are minimal, the ideal gas functions properly. Low pressure and high temperature make this possible.
Hydrogen gas will therefore most likely possess/behave like an ideal gas at 500 K and 0.10 atm.
Consequently, we can say that the conditions under which a sample of neon gas behaves most like an ideal gas ; 500K and 0.1 atm
Learn more about ideal gas at https://brainly.com/question/28257995
#SPJ4
An atomic physicist determines that an atom is composed of 8 positively charged particles and has a mass of 15 amu. Which is the best conclusion that can be drawn?
The atom has 7 neutrons and 8 protons.
The atom has 7 electrons and 8 protons.
The atom has 8 neutrons and 7 electrons.
The atom has 8 electrons and 7 protons.
Answers
Answer:
Explanation:
protons = mass - neutrons
protons are plus so there are 8 protons.
mass = 15
8 = 15 - neutrons
-7 = - neutrons
neutrons = 7
The first two answers are the same and both are correct.
Answer:
a
Explanation:
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
What is the empirical formula of C6H12O6?
C2H4O2
CH2O
CH2O2
C2H4O
Answers
Answer: The empirical formula for C6H12O6 is CH2O. Every carbohydrate, be it simple or complex, has an empirical formula CH2O
Explanation:
