Answers
Answer:
Chemical energy turns into thermal energy.
Explanation:
Related Questions
what organ is very important in human body
Answers
Answer:
The brain is arguably the most important organ in the human body. It controls and coordinates actions and reactions, allows us to think and feel, and enables us to have memories and feelings—all the things that make us human.
Explanation:
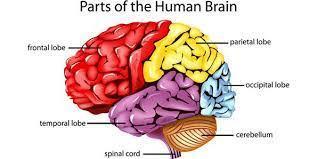
Answer:
The brain is arguably the most important organ in the human body. It controls and coordinates actions and reactions, allows us to think and feel, and enables us to have memories and feelings—all the things that make us human.
Explanation:
how would you confirm the presence of lead in an ore?
Answers
There are numerous ways to determine whether lead is present in an ore. Atomic absorption spectroscopy is a popular approach. With this method, an ore sample is dissolved in acid and then atomized in a flame or plasma.
The sample's atoms will absorb light at particular wavelengths that are peculiar to the element under investigation. The amount of light absorbed can be used to calculate how much lead is present in the sample. Inductively coupled plasma mass spectrometry and X-ray fluorescence spectroscopy are further techniques. It is crucial to remember that these procedures call for specialized tools and training, thus they ought to only be carried out in a lab by qualified experts.
To know more about spectrometry, here
brainly.com/question/31075363
#SPJ1
List the following elements in order of INcreasing number of valence electrons: C, Cl, As, Na, He
Answers
Answer:
He, Cl, As, C, Na
Explanation:
^^
what forms of technology are scientists using to study El Nino
Answers
Answer:
a network of buoys.
Explanation:
It is operated by noaa. The buoys transmit some of the data on a daily basis to NOAA through a satellite in space.
URGENT!!! An unknown hydrate of CoCl₂ has been evaporated in a crucible. Given the following data, find the formula and name of the hydrate.
Mass of crucible: 12.090 g
Mass of hydrate before evaporation and crucible: 16.250 g
Mass of hydrate after evaporation and crucible: 12.424 g
Answers
From the given data, the name of the hydrated salt would be \(CoCl_2.83H_2O\).
Formula of hydrateThe formula of the hydrated salt can be determined using the empirical formula approach. That is, we will find the mole equivalent of the anhydrous salt and the water of hydration and then combine them into a single formula after dividing by the smallest mole.
First, we need to determine the mass of the anhydrous salt and the water of hydration.
Mass of crucible (x) = 12.090 g
Mass of hydrated salt + crucible (y) = 16.250 g
Thus, the mass of the hydrated salt can be determined by subtracting x from y.
Mass of hydrated salt = 16.250 - 12.090 = 4.16 g
Mass of hydrate + crucible after evaporating off the water (z) = 12.424 g
Mass of anhydrous salt = z - x
= 12.424 - 12.090
= 0.334 g
Mass of water = 4.16 - 0.334
= 3.826 g
Now, let's find the moles:
Molar mass of \(CoCl_2\) = 129.839 g/mol
Molar mass of water = 18.01 g/mol
Mole of \(CoCl_2\) = 0.334/129.839 = 0.00257 mol
Mole of water = 3.826/18.01 = 0.2124 mol
Dividing through by the smallest mole
\(CoCl_2\) = 0.00257 / 0.00257 = 1
water = 0.2124/ 0.00257 = 83
Thus, the formula of the hydrate would be \(CoCl_2.83H_2O\)
More on hydrate salts can be found here: https://brainly.com/question/16990374
#SPJ1
How are increasing global temperatures affecting Earth's cryosphere?
Answers
Increasing global temperatures would cause the ice to melt
What is the Earth's cryosphere?
Increasing global temperatures are causing the cryosphere, which includes ice sheets, glaciers, and sea ice, to melt at an accelerated rate. This is leading to rising sea levels, changes in ocean currents, altered weather patterns, and ecosystem disruption.
It is also contributing to a positive feedback loop, as melting ice decreases the amount of sunlight reflected back into space, further warming the planet.
Learn more about earth:https://brainly.com/question/12041467
#SPJ1
22.55 mL of an H2SO4 solution
were titrated with 14.85 mL of a
0.146 M NaOH solution to reach the
equivalence point. What is the
molarity of the H2SO4 solution?

Answers
The concentration of H₂SO₄ solution is equal to 0.0480 M.
What is a neutralization reaction?A neutralization reaction is described as a chemical reaction where acid and base react to produce respective salt and water. When a strong acid reacts with a strong base then the salt can be neutral.
When H₂SO₄ (a strong acid) reacts with NaOH, the resulting salt is Na₂SO₃ and water.
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2H₂O
Given, the concentration of NaOH = 0.146 M
The volume of the NaOH = 14.85 ml = 0.01485 L
The number of moles of NaOH, n = M × V = 0.146 × 0.01485 = 0.00216 M
The volume of the H₂SO₄ = 22.55 ml = 0.02255 L
The number of moles of H₂SO₄, n = 0.00216/2 = 0.00108 mol
The concentration of H₂SO₄ =0.00108/0.02255 = 0.0480 M
Therefore, the molarity of H₂SO₄ is 0.0480 M.
Learn more about neutralization reaction, here:
brainly.com/question/20038776
#SPJ1
What is the mass in grams of
1.0mole of (NH4)2S?
Answers
_________energy from the sun
Answers
Answer:
radiant energy, solar energy
During nuclear fusion, radiant energy and solar energy from the core of the Sun is released. Hope it helps!
Copper reacts with 36.7 g of silver nitrate to produce copper(II) nitrate and silver. Determine the theoretical yield of Cu(NO3)2 (show work)
Answers
The theoretical yield of Cu(NO3)2 is 20.259 grams.
To determine the theoretical yield of Cu(NO3)2, we need to calculate the amount of copper reacting with the silver nitrate and use stoichiometry to convert that amount to the molar mass of Cu(NO3)2.
First, let's find the molar mass of silver nitrate (AgNO3):
AgNO3 = 107.87 g/mol (Ag) + 14.01 g/mol (N) + 3 * 16.00 g/mol (O) = 169.87 g/mol
Next, we need to calculate the amount of copper that reacts with the given mass of silver nitrate. The molar mass of copper (Cu) is 63.55 g/mol.
Using the molar ratio from the balanced chemical equation, we can relate the moles of copper to the moles of silver nitrate. The balanced equation is:
Cu + 2AgNO3 -> Cu(NO3)2 + 2Ag According to the equation, 1 mole of copper reacts with 2 moles of silver nitrate.
Now we can calculate the moles of copper reacting with 36.7 g of silver nitrate:
moles of AgNO3 = (mass of AgNO3) / (molar mass of AgNO3)
moles of AgNO3 = 36.7 g / 169.87 g/mol = 0.2160 mol AgNO3
Using the stoichiometric ratio, we find the moles of Cu:
moles of Cu = (moles of AgNO3) / (2 moles of AgNO3 per 1 mole of Cu)
moles of Cu = 0.2160 mol AgNO3 / (2 mol AgNO3/1 mol Cu) = 0.1080 mol Cu
Finally, we can calculate the theoretical yield of Cu(NO3)2 using the molar mass of Cu(NO3)2:
theoretical yield of Cu(NO3)2 = (moles of Cu) * (molar mass of Cu(NO3)2)
theoretical yield of Cu(NO3)2 = 0.1080 mol * (63.55 g/mol + 2 * (14.01 g/mol + 3 * 16.00 g/mol))
theoretical yield of Cu(NO3)2 = 0.1080 mol * 187.56 g/mol
theoretical yield of Cu(NO3)2 = 20.259 g
For more such questions on theoretical yield visit:
https://brainly.com/question/14714924
#SPJ11
A solution of aluminum chloride has a pH of (4.5x10^0). What is the [H3O*(aq)], in mol/L?
Note: Your answer is assumed to be reduced to the highest power possible.
Answers
The concentration of H3O+ ions in the solution of aluminum chloride is \(3.16×10^-5\) mol/L.
Aluminum chloride is an acidic salt that contains a cation, Al3+, and an anion, Cl-. When aluminum chloride is dissolved in water, it dissociates into its constituent ions, and the Al3+ cations hydrolyze to produce H+ ions.
This reaction leads to the formation of an acidic solution. The pH of a solution of aluminum chloride is \(4.5×10^0\). We need to determine the concentration of H3O+ ions in this solution.
The concentration of H3O+ ions in a solution is given by the equation: pH = -log[H3O+] where pH is the negative logarithm of the concentration of H3O+ ions in the solution. The negative sign indicates that the pH is inversely proportional to the concentration of H3O+ ions. To determine the concentration of H3O+ ions, we need to rearrange the equation:
[H3O+] = \(10^-pH\) Substituting the value of pH =\(4.5×10^0\), we get: [H3O+] = \(10^-4.5\)
The value of \(10^-4.5\) can be calculated using scientific notation: \(10^-4.5\)= \(3.16×10^-5\) mol/L
for more such questions on concentration
https://brainly.com/question/28564792
#SPJ8
PLEASE HELP QUICKK
Calculate the energy of combustion for one mole of butane if burning a 0.367 g sample of butane (C4H10) has increased the temperature of a bomb calorimeter by 7.73 °C. The heat capacity of the bomb calorimeter is 2.36 kJ/ °C.
Answers
The energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
To calculate the energy of combustion for one mole of butane (C4H10), we need to use the information provided and apply the principle of calorimetry.
First, we need to convert the mass of the butane sample from grams to moles. The molar mass of butane (C4H10) can be calculated as follows:
C: 12.01 g/mol
H: 1.01 g/mol
Molar mass of C4H10 = (12.01 * 4) + (1.01 * 10) = 58.12 g/mol
Next, we calculate the moles of butane in the sample:
moles of butane = mass of butane sample / molar mass of butane
moles of butane = 0.367 g / 58.12 g/mol ≈ 0.00631 mol
Now, we can calculate the heat released by the combustion of the butane sample using the equation:
q = C * ΔT
where q is the heat released, C is the heat capacity of the calorimeter, and ΔT is the change in temperature.
Given that the heat capacity of the bomb calorimeter is 2.36 kJ/°C and the change in temperature is 7.73 °C, we can substitute these values into the equation:
q = (2.36 kJ/°C) * 7.73 °C = 18.2078 kJ
Since the heat released by the combustion of the butane sample is equal to the heat absorbed by the calorimeter, we can equate this value to the energy of combustion for one mole of butane.
Energy of combustion for one mole of butane = q / moles of butane
Energy of combustion for one mole of butane = 18.2078 kJ / 0.00631 mol ≈ 2888.81 kJ/mol
Therefore, the energy of combustion for one mole of butane is approximately 2888.81 kJ/mol.
In conclusion, by applying the principles of calorimetry and using the given data, we have calculated the energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
For more questions on molar mass, click on:
https://brainly.com/question/837939
#SPJ8
Match the scientist with their scientific idea.
Substances combine or
break apart to create new,
different substances.
1. Democritus
2. Bernoulli
Gases are formed from tiny
particles so small you can't
see them. The particles are
spread out into a certain
area and move when
people walk through them.
3. Priestley
Matter was made of
different kinds of things.
Answers
Answer:
1. Democritus- Matter was made of
different kinds of things.
2. Bernoulli- Gases are formed from tiny particles so small you can't see them. The particles are spread out into a certain area and move when people walk through them.
3. Priestley- Substances combine or
break apart to create new different substances.
Explanation:
1. Democritus was a Greek philosopher regarded to be the "Father of Science". He discovered that all matter were composed of indestructible things he called ATOMOS. Hence, his idea was that Matter was made of different kinds of things (atoms).
2. David Bernoulli, a mathematician, was born in 1700. He contributed immensely to the kinetic molecular theory of gases. In his postulated theory, he gave the idea that gases are formed from tiny particles so small you can't see them. The particles are spread out into a certain area and move when people walk through them.
3. Priestley- Joseph Priestley, born in 1733, is widely known for his discovery of oxygen gas among other gases. His idea was that substances combine or
break apart to create new different substances.
Which image shows 2-hexyne?

Answers
Answer: C
Explanation:
A. Shows 3-Hexyne (NOT 2-HEXYNE)
B. Shows 7 carbons (too many) (NOT 2-HEXYNE)
C. Shows a triple bond (yne) and 6 carbons and it's on the second carbon (2-HEXYNE)
D. Shows two substitent on the second carbon but the triple bond is on the 3rd carbon so it's 2,2-dimethyl-3-heptyne (NOT 2-HEXYNE)
when liquid water loses energy, a change in state or chemical identity occurs?
Answers
Answer:
When most substances lose or gain energy, one of two things happens to the substance: its temperature changes or its state changes. The temperature of a substance is related to the speed of the substance’s particles. So, when the temperature of a substance changes, the speed of the particles also changes. But the temperature of a substance does not change until the change of state is complete.
Explanation:
hope this helps,
noah
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. Drag each item to the appropriate bin. HCl, NaOH, HC2H3O2, HF, C2H5OH, HNO3, C6H12O6 Strong Electrolytes Weak Electrolytes Nonelectrolytes
Answers
Powerful Electrolytes:
HCl, NaOH, HNO3
Weak electrolyte:
HF, HC2H3O2
• Non-electrolytes:
C2H5OH, C6H12O6
Further explanation
An electrolyte solution is a substance that produces ions when dissolved in water and can conduct electricity.
Strong electrolytes are the solution
Solutes have the strongest electrical conductivity because they are completely ionized when dissolved in water.
• Weak electrolytes are partially ionized solutions with low electrical conductivity.
• Non-electrolytes are solutions that cannot conduct electricity because the solute cannot form ions. One of the most important properties of water is its ability to dissolve various substances. Solutions in which water is actually the dissolution medium are called aqueous solutions. Water is the most important solvent for electrolytes.
HCl = hydrochloric acid, a strong acid.
HNO3 = nitric acid, strong acid
• NaOH = sodium hydroxide, a strong base
HF = hydrofluoric acid, a weak acid
HC2H3O2 or CH3COOH = acetic acid, a weak acid
C2H5OH = ethanol, non-electrolyte
C6H12O6 = glucose, non-electrolyte
remarks:
• Some acids are fully ionized in water, while others are partially ionized. Not all acids are equally strong in generating H+ ions in solution. When an acid is fully ionized it is a strong acid. Click here when passing through hydrogen chloride
learn more about as a electrolyte ;
https://brainly.com/question/17089766
#SPJ4
calculate the number of carbon hydrogen and oxygen atoms in 1.5 gram of glucose
Answers
The number of atoms :
C = 6 x 5 x 10²¹ = 3 x 10²²
H = 12 x 5 x 10²¹ = 6 x 10²²
O = 6 x 5 x 10²¹ = 3 x 10²²
Further explanationGiven
1.5 g glucose(C₆H₁₂O₆)
Required
The number of atoms
Solution
mol of glucose(MW=6.12+12.1+6.16=180 g/mol) :
mol = mass : MW
mol = 1.5 : 180
mol = 0.0083
Number of molecules = 0.0083 x 6.02 x 10²³ = 5 x 10²¹
There are 6 C, 12 H and 6 O, so number or atoms :
C = 6 x 5 x 10²¹ = 3 x 10²²
H = 12 x 5 x 10²¹ = 6 x 10²²
O = 6 x 5 x 10²¹ = 3 x 10²²
What is one of the major conclusions made from the study of line spectra of various elements?
A)
the movement of electrons outside the nucleus
B)
absorption and emission of energy is quantized
C)
the presence of energy beyond the visible spectrum
D)
the presence of electrons outside the nucleus of an atom
Answers
Answer:
B
Explanation:
proceeds from notes payable on january 26, nyree co. borrowed cash from conrad bank by issuing a 45-day note with a face amount of $225,000. assume a 360-day year. a. determine the proceeds of the note, assuming the note carries an interest rate of 8%. $fill in the blank 1 b. determine the proceeds of the note, assuming the note is discounted at 8%. $fill in the blank 2
Answers
The revenues of the note will equal the $225,000 face value, provided the note has an interest rate of 8%.
The interest rate today is what.On Friday, December 9, 2022, the benchmark 30-year fixed mortgage's average rate is 7.32%, which is a 15 basis point increase from the previous week. If you want to restructure your present mortgage.
The nominal value and face value are both used interchangeably. That which a money is worth. Simply said, it is a security's nominal value. A 45-day note having a face value of $225,000 was issued by Nyree Co. to Conrad Bank based on the information provided. Consequently, the amount of the note's revenues will match its face value, which is 225,000.
To know more about interest rate visit:-
https://brainly.com/question/13324776
#SPJ4
What limitations occurs for chalk in vinegar chemistry pd lab experiment?
Also the precautions to take
Need this asap!!
Answers
Answer:
When conducting a chemistry lab experiment using chalk (calcium carbonate) in vinegar (acetic acid), there are several limitations and precautions to be aware of:
Limitations of chalk in vinegar chemistry experiment:
Reaction rate: The reaction between chalk and vinegar is relatively slow, which may require a longer observation period or higher concentration of vinegar to observe significant changes within a reasonable time frame.
Solubility: Chalk may not dissolve completely in vinegar, resulting in incomplete reaction or difficulty in obtaining accurate results.
Product formation: The reaction between chalk and vinegar produces carbon dioxide gas, water, and calcium acetate. The carbon dioxide gas may escape into the atmosphere, leading to loss of product and inaccurate measurements.
pH: Chalk is a basic substance, and the reaction with vinegar, which is acidic, may result in neutralization, leading to a decrease in the overall acidity of the reaction mixture.
Precautions to take in chalk in vinegar chemistry experiment:
Ventilation: The reaction between chalk and vinegar produces carbon dioxide gas, which can displace air and potentially cause asphyxiation in a closed or poorly ventilated area. Conduct the experiment in a well-ventilated area or under a fume hood to ensure adequate air circulation.
Eye and skin protection: Vinegar is an acid and can cause skin and eye irritation. Wear appropriate personal protective equipment (PPE), such as gloves and goggles, to protect yourself from contact with vinegar or any other chemicals used in the experiment.
Chemical handling: Handle the chemicals, including chalk and vinegar, with care, following proper lab safety protocols. Avoid ingestion, inhalation, or direct contact with the chemicals, and dispose of them properly according to local regulations.
Accuracy in measurements: Use calibrated and accurate measuring tools, such as graduated cylinders or burettes, to measure the amount of chalk, vinegar, and other reagents accurately. This will ensure the reliability and accuracy of the experimental results.
Observations: Make careful and detailed observations during the experiment, noting any changes in appearance, gas evolution, or other relevant observations. Take measurements at appropriate intervals and record the data accurately for analysis and interpretation.
It is important to follow good laboratory practices, including proper chemical handling, accurate measurements, and cautious observations, to ensure safe and reliable results in a chalk in vinegar chemistry lab experiment. Consult with a qualified instructor or supervisor for specific guidelines and precautions related to your experiment.
A student observes a chemical
reaction where two liquids are mixed together. After the liquids are mixed the beaker feels cold to touch. This reactions is an
example of a
reaction.
Synthesis
Combustion
Exothermic
Endothermic
Answers
Answer:
Endothermic
Explanation:
Endothermic reaction is one in which the enthalpy increases. What this implies is that, it is a closed system which absorbs heat from its surroundings and thus after reaction become cooler than prior to the reaction.
In this case, the mixture of the two liquids feel colder to touch. Thus, it is an endothermic reaction from the definition earlier given.
An electron in the first energy level of the electron cloud has
an electron in the third energy level.
Answers
Answer: A LOWER ENERGY
Explanation:
did the test
Answer:
A lower energy!
Explanation:
I know this is not much of an explanation but I took the test i hope you have an amazing day afternoon or night! Hope this helps :3
6. Observe the reaction below and choose the best answer which completes the
reaction.
C-C=C + HOH ===> ?
(will you be able to determine the answer?)
Answers
Answer:
The answer to this reaction would be C-C-OH + H2.
(01.01 LC)What is the body of scientific knowledge based on?
Guesses
Mysteries
Observations
Opinions
Answers
The body of scientific knowledge is based on different Observations (Option C).
What does observations mean in the scientific method?Observations in the scientific method are fundamental because it is the first step to raising scientific questions that may be explained through plausible hypotheses. Subsequently, hypotheses must be tested by experimental procedures.
In conclusion, the body of scientific knowledge is based on different Observations (Option C).
Learn more about observations in the scientific method here:
https://brainly.com/question/2505873
#SPJ1
A compound is 60.00% carbon, 5.75% hydrogen, and 34.25% oxygen. Determine the empirical formula of the compound.
Answers
Answer:
The empirical formula of the compound is C2H5O.
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
classify each of the following as a pure substance or a mixture.
a) baking soda
b) ice
c)blueberry muffin
d) zink
Answers
Ice- purse substance
Blueberry muffin- mixture
Zink- mixture
Answer:
c
Explanation:
its c because it has multiple mixture blueberries flower water and others thats why i says c
Please select the word from the list that best fits the definition
Represents a range of responses to a question:
Caption
Legend/Key
Scale
Answers
Answer:
The answer is B(scale)
Explanation:
Since the question asked about a range, the scale would be the most logical answer since scales are used to measure.
Answer:
the answer is C.
Explanation:
is the nucleus of these atoms positive negative or neutral
Answers
Answer: they are Positively charged
What mass of carbon dioxide is produced from the complete combustion of 5.30x10-3 g of methane?
Express your answer with the appropriate units.
View Available Hints
Answers
Answer:
1.45 x 10⁻² g CO₂
Explanation:
To find the mass of carbon dioxide, you need to (1) convert grams CH₄ to moles CH₄ (via molar mass), then (2) convert moles CH₄ to moles CO₂ (via mole-to-mole ratio from reaction coefficients), and then (3) convert moles CO₂ to grams CO₂ (via molar mass). The final answer should have 3 sig figs to reflect the given value (5.30 x 10⁻³ g).
Molar Mass (CH₄): 12.011 g/mol + 4(1.008 g/mol)
Molar Mass (CH₄): 16.043 g/mol
Combustion of Methane:
1 CH₄ + 2 O₂ ---> 2 H₂O + 1 CO₂
Molar Mass (CO₂): 12.011 g/mol + 2(15.998 g/mol)
Molar Mass (CO₂): 44.007 g/mol
5.30 x 10⁻³ g CH₄ 1 mole 1 mole CO₂ 44.007 g
--------------------------- x ---------------- x --------------------- x ----------------- =
16.043 g 1 mole CH₄ 1 mole
= 0.0145 g CO₂
= 1.45 x 10⁻² g CO₂