a water molecule can bond to up to other water molecules by bonds. a water molecule can bond to up to other water molecules by bonds. two ... hydrogen three ... ionic four ... polar covalent two ... polar covalent four ... hydrogen
Answers
A water molecule can bond to up to 3 other water molecules by hydrogen bonding.
What is hydrogen bond ?A hydrogen bond, also known as an H-bond, is an attraction that is essentially electrostatic and occurs when an electronegative atom with a single pair of electrons, or the hydrogen bond acceptor, is covalently attached to a greater electronegative "donor" atom or group (Dn) (Ac). A polar covalent bond is often denoted by a solid line, while a hydrogen bond is denoted by a dotted or dashed line. Such an interacting system is generally represented by the symbols DnHAc. The second-row elements nitrogen (N), oxygen (O), and fluorine (F) are used as donors and acceptors the most frequently (F).Intermolecular (between different molecules) or intramolecular (occurring among parts of the same molecule) hydrogen bonds are two different types of hydrogen bonding .Learn more about Hydrogen bond with the given link
https://brainly.com/question/16854739
#SPJ4
Related Questions
draw the structure of the product of the reaction of propylmagnesium bromide with benzaldehyde. assume that the reaction is worked up by the addition of dilute aqueous acid. click the draw structure button to launch the drawing utility.
Answers
The final product of the reaction of propylmagnesium bromide with benzaldehyde is phenol component with side product. The above figure show the reaction.
A Grignard reaction is a reaction in which alkyl/aryl magnesium halide reacts with a carbonyl group, C=O. This reaction has been named on the name of Victor Grignard. We have to draw the structure of the product of the reaction of propylmagnesium bromide with benzaldehyde. The chemical formula for Propylmagnesium bromide is CH₃CH₂CH₂MgBr and Benzaldehyde is C₆H₅CHO. When benzaldehyde reacts with grignard reagent in dilute aqueous acid ( H positive). It will produce adduct alcohol that is not a product. In between final product, reagent attached to benzene ring with Oxygen site but it is not stable, so, the bonds break up and MgBr removed by leaving Hydrogen in its place. So, the final product is Phenol with side product CH₃CH₂CH₂CH₃.
For more information about grignard reaction, visit :
https://brainly.com/question/23971610
#SPJ4

what is the molality of a solution that contains 5.2 g of urea
Answers
The molality of a solution containing 5.2 g of urea is 0.56 m (molar mass = 60 g/mol) in 250 g of benzene (\(C_{6}H_{6}\)) is 0.35 m.
We are given:
The mass of urea is 5.2 gThe molar mass of urea is 60 g/molThe mass of benzene is 250 gUsing the mass and molar mass of urea, the number of moles of urea is:
\(n_{urea}\) = mass of urea ÷ molar mass of urea
= 5.2 g ÷ 60g/mol
= 0.087 mol
The molality of the solution is expressed as follows:
Molality = moles of urea ÷ kilogram of benzene
Now solving the above equation and gives:
Molality = (0.087 mol ÷ 250 g) × (1000 g ÷ 1 kg)
= 0.348 mol/kg
≈ 0.35 m
Hence, the molality of the solution is 0.35 m.
Your question is incomplete, but most probably your full question was
What is the molality of a solution that contains 5.2 g of urea (molar mass = 60 g/mol) in 250 g benzene (\(C_{6}H_{6}\))?
For more information about molality refers to the link: https://brainly.com/question/26921570
#SPJ11
Calculate the root mean square velocity for the atoms in a sample of helium gas at 25°C
Answers
The gas constant is equal to R=8.314 J/mol K, while the atomic mass of helium is equal to M=4.0 g/mol. M = 4.0 g / m o l . As a result, the helium atom's rms speed is 1304.7 m/s. So, 515 m/sec is the root-mean-square speed.
What is root mean square velocity?The value of the square root of the sum of the squares of the stacking velocity values divided by the quantity of values is the root-mean square (RMS) velocity. The RMS velocity is the speed of a wave traveling along a certain ray path across subsurface strata with various interval velocities.
The expression v=sqrt(3RTM) and v=sqrt(3KTm), where R is the universal gas constant, T is the absolute (Kelvin) temperature, m is the molar mass, K is the Boltzmann's constant, and M is the molecular mass, gives the root mean square (R.M.S.) speed V of the molecules of an ideal gas.
Since the particles in a typical gas sample are flowing in all directions, the average velocity for that sample is zero, which is why we use the rms velocity instead of the average. This is an important formula since the particle velocity controls both the diffusion and effusion rates.
To know more about root mean square velocity refer to:
https://brainly.com/question/15995507
#SPJ13
The resistance of a thermometer is 5 ohm at 25 degree Celsius and 6 2 at 50 degree Celsius. Using linear approximation, calculate the value of resistance temperature coefficient at 45 degree Celsius.
Answers
The approximate resistance value at 45 degrees Celsius is around 5.8 ohms.
To calculate the value of the resistance temperature coefficient at 45 degrees Celsius using linear approximation, we can use the formula:
R(T) = R0 + α(T - T0),
where R(T) is the resistance at temperature T, R0 is the resistance at a reference temperature T0, α is the resistance temperature coefficient, and (T - T0) is the temperature difference.
Given that the resistance at 25 degrees Celsius is 5 ohms (R0 = 5) and the resistance at 50 degrees Celsius is 6 ohms (R(T) = 6), we can calculate the value of α.
6 = 5 + α(50 - 25),
Simplifying the equation:
1 = 25α,
Therefore, α = 1/25 = 0.04 ohm/degree Celsius.
Using the linear approximation, we can approximate the value of the resistance at 45 degrees Celsius:
R(45) = 5 + 0.04(45 - 25) = 5 + 0.04(20) = 5 + 0.8 = 5.8 ohms.
Therefore, the value of the resistance at 45 degrees Celsius is approximately 5.8 ohms.
You can learn more about resistance at
https://brainly.com/question/17563681
#SPJ11
please help me out! which statement is true?
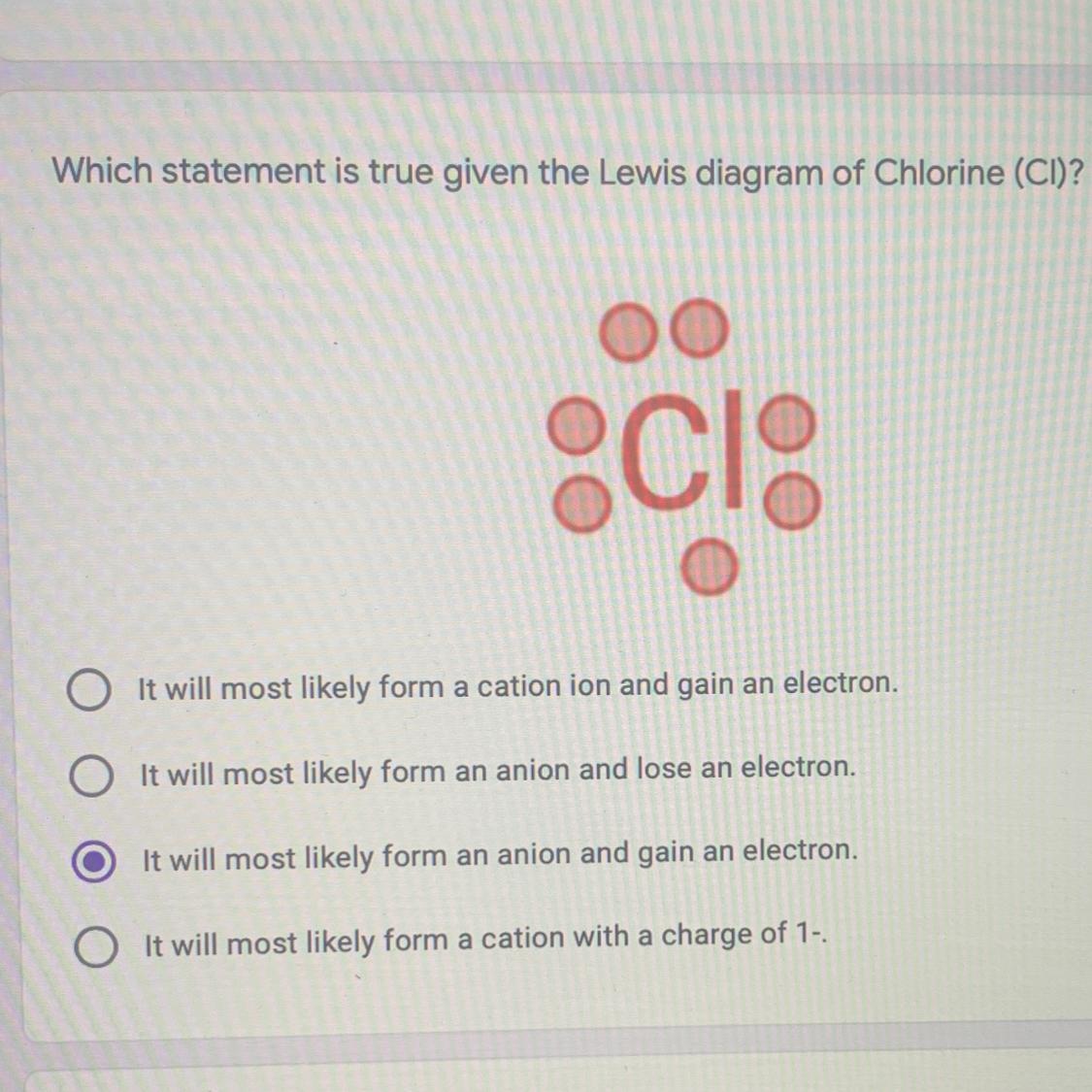
Answers
1st one
Explanation:
cause it has 17 proton number nd to complete the diagram it gain 1 electron so
help please please please please please

Answers
Answer:
wow only six points, you have to amp it up a little
Explanation: because
The number of protons in this element is __________.
8
2
4
6

Answers
Answer: 2 protons
Explanation:
The element number, 2 for He, is the number of protons in the nucleus. The 4.0026 is the average atomic mass of He.
Julie tears a piece of notebook paper. This is a(n) _____.
Answers
please help!!
Strontium-90 has a half-life of 28.8 years. If you start with a 10-gram sample of strontium-90, how much will be left after 115.2 years? Justify your answer.
Answers
The amount that will be left after 115.2 years, given that you started with 10 grams sample of strontium-90 is 0.625 g
How do I determine the amoun remaining after 115.2 years?To obtain the amount that will be left after 115.2 years, we shall begin our calculation by obtaining the number of half lives that has passed.
The number of half lives that has passed can be obtained as followed:
Half-life (t½) = 28.8 yearsTime (t) = 2 months = 115.2 yearsNumber of half-lives (n) =?n = t / t½
n = 115.2 / 28.8
n = 4
Thus, 4 half lives has passed.
Finally, we shall determine the amount that will be left after 115.2 years. This is illustrated below:
Number of half-lives (n) = 4Original amount (N₀) = 10 gAmount remaining (N) = ?N = N₀ / 2ⁿ
N = 10 / 2⁴
N = 10 / 16
N = 0.625 g
Thus, we can conclude that the amount that will be left after 115.2 years is 0.625 g
Learn more about amount remaining:
https://brainly.com/question/28440920
#SPJ1
Place each scenario under the correct type of friction
swimming
Static Friction
Kinetic Friction
Fluid Friction
books on a desk
riding a bike
skydiving
moving furniture
a parked car
Answers
Answer:
static friction: books on a desk and a parked car
kinetic friction: moving furniture and riding a bike
fluid friction: swimming and skydiving
Explanation:
Answer:
static friction:
books on a desk a parked carkinetic friction:
moving furniture riding a bikefluid friction:
swimming skydivingpls help!!!!!!!!!!!!!!

Answers
The given reaction is the calcium carbide reaction of calcium hydroxide with water to produce acetylene.
How much C2H2 AND Ca(OH)2 was produced?Chemical equations must obey the law of conservation of mass and the law of constant proportions. That is, the reactant and product sides of the equation must contain the same number of atoms of each element or compound.The given reaction is the calcium carbide reaction of calcium hydroxide with water to produce acetylene.The balanced equation is CaC2+2H2OCa(OH)2+C2H2.
Molar mass of acetylene, C2H2 = 2x12+2x1 = 26 g/mol
we get 64 g of CaC2 becomes 26 g of C2H2. 5.0g CaC2..
Molecular weight of Ca(OH)2=1(atomic weight of Ca)+2(atomic weight of O)+2(atomic weight of H)=40+2(16)+2(1)=40+32+2=74.
For more information on Molecular weight, see:
https://brainly.com/question/27988184
#SPJ1
the lattice energy of sodium chloride, nacl, is -787.5 kj/mol. the lattice energy of potassium chloride, kcl, is -715 kj/mol. in which compound is the bonding between ions stronger? why?
Answers
Because NaCl takes more energy to break it's bigger.
For NaCl, the structure or lattice formation enthalpy is -787 kJ mol-1. Therefore, the lattice dissolution enthalpy is always positive and the lattice formation enthalpy is always negative. The reason that NaCl has the highest lattice enthalpy is that for similar anions, cations with smaller radii have higher lattice enthalpies.
If we want to talk about the amount of energy released when a lattice forms from scattered gas ions we should use the lattice formation enthalpy. MgO has a higher lattice energy because each ion carries two unit charges compared to one in NaCl. NaCl is a compound with higher lattice energy. Description Lattice energy is defined as the energy released when the combination of its constituent ions forms one mole of an ionic compound.
Learn more about The lattice energy here:-https://brainly.com/question/2815368
#SPJ4
a 10 ml sample of 0.20 m hbr solution is titrated with 0.10 m naoh. what volume of naoh is required to reach the equialvence point?
Answers
The volume Naoh is required to reach the equivalent point is L.
Equivalent point is the point in the chemical reaction when there is acid or base to neutralize the solution. It is also known as stoichiometric point. The moles of the titrant is equal to the moles of the unknown concentration in the titration.
The volume of the nbr solution is 0.20m and the concentration of nbr is 10ml. and concentration of the Noah is 0.10.so, here C1 is 0.20m and v1 is 0.010l and C2 is 0.10m .
so putting all the value sin the expression,
C1 V1 = C2 V2
where C1 is the concentration of the acid ,c2 is the concentration of the base,V1 is the volume of the acid and V2 is the volume of the base.
V2=C1. V1 / C2
= 0.20 .0.010 /0.10
= L of Naoh.
To learn more about Equivalent point in titration please visit:
https://brainly.com/question/2496608
#SPJ4
helpppp with this chemistry workkkk

Answers
Answer:
The molecules that go are go crazy so that there are a lot more positive of than negative
Explanation:
I REALLY REALLY REALLY HOPE THIS HELPS PLEASE
A man weighs 181 pounds. How much is this in kilograms?
Answers
Answer:
82.1 kilograms
Explanation:
divide 181 by 2.205 to convert
In an experiment, 8.50 g of methane, CH4, was reacted with 15.9 g of oxygen gas to produce carbon dioxide and water. Determine the percentage yield if 9.77 g of carbon dioxide was obtained in the lab.
Answers
Answer:
89.3 %
Explanation:
M(CH4) = 12+ 4*1 = 16 g/mol
M(O2) = 2*16 = 32 g/mol
M(CO2) = 12 + 2*16 = 44 g/mol
8.50 g * 1 mol/16 g = 0.5313 mol CH4
15.9 g * 1 mol/32 g = 0.4969 mol O2
9.77 g * 1 mol/44 g = 0.2220 mol CO2
1) CH4 + 2O2 -----> CO2 + 2H2O
from reaction 1 mol 2 mol
given 0.5313 mol (0.4969 mol)
1 mol CH4 --- 2 mol O2
0.5313 mol CH4 --- x mol O2
x= 2*0.5313 = 1.0626 mol O2
We can see that for given amount of CH4 we do not have enough O2, so O2 is a limiting reactant.
2) CH4 + 2O2 -----> CO2 + 2H2O
from reaction 2 mol 1 mol
given 0.4969 mol x mol
x = 0.4969*1/2 = 0.2485 mol CO2 theoretical yield
3)
Practical yield CO2 = 0.2220 mol
Theoretical yield CO2 = 0.2485 mol
% yield = (0.2220/0.2485)*100% = 89.3 %
Calculate the volume of 100. grams of oxygen gas (O₂) at 25.0°C and a pressure of 1.25 atm.
Answers
Ideal gas law states
PV = n(RT)V is volume (L), P is pressure (atm), R is universal gas constant: 0.0821 L·atm/mol· K, n is number of moles, T is temperature in Kelvin
Here given:
\(\blacksquare\) mass of O₂ : 100 gram
\(\blacksquare\) moles: mass/molar mass = 100/32 = 3.125 moles
\(\blacksquare\) 25.0 °C = (25+273.15) = 298.15 K
\(\blacksquare\) pressure: 1.25 atm
Volume:
\(\boxed{\sf V=\dfrac{nRT}{P}}\)
\(\Rightarrow \sf V=\dfrac{(3.125 \ x \ 0.0821 \ x \ 298.15 )}{1.25}\)
\(\Rightarrow \sf V=61.195 \ L\)
\(\Rightarrow \sf V=61.2 \ L \ \ \ (rounded \ to \ nearest \ tenth)\)
Additional Explanation:
Volume can be counted in multiple units.In Liters: 61.2 Liters
In cubic meter: 0.061163 m³
In cubic centimeter: 61,163.5 cm³
Suppose you have 56. 8 g of sulfur (S), how many moles of sulfur do you have? (4 points)
Answers
If you have 56. 8 g of sulfur (S), then probably you have approximately 1.772 moles of sulfur.
To determine the number of moles of sulfur (S) from the given mass, first of all you need to divide the given mass by the molar mass of sulfur.
The molar mass of sulfur (S) is approximately 32.06 g/mol.
Using the given mass of sulfur:
Moles of sulfur (S) = Mass of sulfur / Molar mass of sulfur
Moles of sulfur (S) = 56.8 g / 32.06 g/mol
Moles of sulfur (S) ≈ 1.772 mol
Therefore, you have approximately 1.772 moles of sulfur.
Learn more about Molar mass from the link given below.
https://brainly.com/question/31545539
#SPJ4
if rates of nitrogen fixation increased tenfold in aquatic ecosystems, would you expect a tenfold increase in primary productivity?
Answers
It is not necessarily a straightforward relationship that a tenfold increase in nitrogen fixation would lead to a tenfold increase in primary productivity in aquatic ecosystems.
There are a few different factors that likewise assume a part in deciding primary productivity, like the accessibility of different supplements, light, temperature, and water conditions.
Moreover, an expansion in nitrogen obsession might prompt an expansion in primary productivity at first, however as the ecosystem acclimates to the new degrees of nitrogen, the relationship might change.
Hence, it is hard to make a conclusive forecast on the effect of a ten times expansion in nitrogen obsession with primary productivity in aquatic ecosystems disregarding these different factors and the potential criticism mechanisms included.
To know more about aquatic ecosystems:
https://brainly.com/question/17246719
#SPJ4
Energy in vs Energy out = Energy balance. Explain this concept, give examples and provide support for your explanation.
Answers
The concept of energy balance refers to the equilibrium between the energy input into a system and the energy output from that system. It is based on the principle of conservation of energy, which states that energy cannot be created or destroyed but can only be transferred or transformed from one form to another.
In terms of human energy balance, it involves the energy intake from food and beverages (energy in) and the energy expenditure through basal metabolic rate, physical activity, and other bodily processes (energy out). When the energy intake matches the energy expenditure, there is an energy balance. However, when there is an imbalance, either an excess or deficit of energy, it can lead to weight gain or weight loss, respectively.
For example, if a person consumes 2000 calories (energy in) through their diet and expends 2000 calories (energy out) through their daily activities and bodily functions, they maintain an energy balance. This means that the energy intake is equal to the energy expenditure, and their weight remains stable.
On the other hand, if a person consumes 2500 calories (energy in) but only expends 2000 calories (energy out), there is a positive energy balance. The excess energy is stored in the body as fat, leading to weight gain over time.
Conversely, if a person consumes 1500 calories (energy in) but expends 2000 calories (energy out), there is a negative energy balance. The body needs to compensate for the energy deficit by utilizing stored energy reserves, such as fat, resulting in weight loss.
Support for the concept of energy balance comes from scientific studies on weight management and obesity. It has been shown that maintaining an energy balance is crucial for weight maintenance, while sustained positive or negative energy balances can lead to weight changes. Additionally, energy balance plays a role in various physiological processes, including metabolism, hormone regulation, and overall health.
By understanding and managing energy balance, individuals can make informed decisions regarding their diet, physical activity, and lifestyle to achieve and maintain a healthy weight and overall well-being.
To know more about energy balance, click here, https://brainly.com/question/31922451
#SPJ11
what is the function of a prism
Answers
Answer:
Prisms are sometimes used for the internal reflection at the surfaces rather than for dispersion. If light inside the prism hits one of the surfaces at a sufficiently steep angle, total internal reflection occurs and all of the light is reflected.
Explanation:
Prism, in optics, piece of glass or other transparent material cut with precise angles and plane faces, useful for analyzing and reflecting light. An ordinary triangular prism can separate white light into its constituent colours, called a spectrum.
5. in this experiment, 1.0 ml of saturated sodium chloride is used to rinse the hickman head after the initial distillation. why is saturated sodium chloride, rather than pure water, used for this procedure and the subsequent washing of the organic layer?
Answers
Saturated sodium chloride is used in this procedure because it helps to separate the organic layer from the aqueous layer.
What is organic layer?
Organic layer is a type of soil layer that includes organic matter such as leaves, wood, roots, and other plant and animal remains. This type of soil is important for agricultural production and is also beneficial to the environment. Organic layer is also known as top soil or humus. It is the most fertile and nutrient rich soil layer, due to the presence of the decomposing organic matter. Organic layer helps to regulate soil temperature and moisture, improve soil structure and aeration, and provide essential nutrients for plant growth. It also helps to reduce erosion, improve water-holding capacity, and keep soil particles in place. Organic layer also helps to increase the biodiversity of the soil, as it provides a habitat for beneficial microorganisms and other organisms. Organic layer is an important part of any healthy soil system.
The sodium chloride solution has a higher density than water and helps to ensure that the two layers remain distinct. Additionally, the sodium chloride helps to break the surface tension between the two layers, making it easier to separate them.
To learn more about organic layer
https://brainly.com/question/14356327
#SPJ4
How can you demonstrate the helium takes up space?
Answers
What is the standard enthalpy of the reaction CO+H2O --> CO2+H2?
Answers
The standard enthalpy of the reaction CO + H2O → CO2 + H2 is -679.3 kJ/mol. The standard enthalpy of the reaction CO + H2O → CO2 + H2 can be determined by calculating the difference in the standard enthalpies of formation between the products and the reactants.
The enthalpy change for this reaction is -41.2 kJ/mol.
The standard enthalpy of a reaction is the difference in the standard enthalpies of formation between the products and the reactants. The standard enthalpy of formation is the enthalpy change when one mole of a compound is formed from its constituent elements in their standard states.
To determine the standard enthalpy of the reaction CO + H2O → CO2 + H2, we need to subtract the sum of the standard enthalpies of the formation of the reactants from the sum of the standard enthalpies of the formation of the products.
The standard enthalpy of the formation of CO2 is -393.5 kJ/mol, and the standard enthalpy of the formation of H2O is -285.8 kJ/mol. The standard enthalpies of the formation of CO and H2 are zero since they are elements in their standard states.
Using these values, we can calculate the standard enthalpy of the reaction:
Standard enthalpy of reaction = Σ(standard enthalpies of formation of products) - Σ(standard enthalpies of formation of reactants)
Standard enthalpy of reaction = [(-393.5 kJ/mol) + (-285.8 kJ/mol)] - [0 + 0]
Standard enthalpy of reaction = -679.3 kJ/mol
To learn more about standard enthalpy, refer:-
https://brainly.com/question/28303513
#SPJ11
Which will most likely happen to her soup?
Carlotta adds too much salt to her soup. She recalls that
evaporation can be used to separate salt from salt water,
so she plans to leave the soup on the stove on low heat
until the soup is less salty.
O The soup will become saltier because evaporation
removes the water and leaves the salt behind.
O The procedure will make the soup less salty because
the salt will evaporate and leave the pot.
The saltiness of the soup will not change because
both salt and water will evaporate.
O The heat will cause the soup to become more salty
because more salt dissolves in hotter water.
Answers
Answer: A! The soup will become saltier because evaporation removes the water and leaves the salt behind.
Explanation:
Just finished the test and got a 90%! <3
Answer:
A
Explanation:
7.8 L =mLDimensional AnalysisRatio:ProportionFormula MethodmLХLx mL=IImL11
Answers
answer and explanation
1 L = 1000 mL
and so to determine 7.8L we can o the calculation as follows
x mL = 1000 mL/ 1 L x 7.8 L/1 = 7800 mL/1 = 7800 mL
What is the mass of 8.03 mole of NH3? (NH3 is 17g/mol)
Answers
Answer:
the mass of 8.03 mole of NH3 is 136.51 g
Explanation:
The computation of the mass is shown below:
As we know that
Mass = number of moles × molar mass
= 8.03 mol × 17 g/mol
= 136.51 g
Hence, the mass of 8.03 mole of NH3 is 136.51 g
We simply multiplied the number of moles with the molar mass so that the mass could come
To shape, form, or improve a metal through compression or stretching is to?
Answers
The process by which we pass a metal to shape, form, or improve a metal through compression or stretching is the process that we refers to as forging the metal.
What is metal forging?We know that metals can be used for a number of purposes. In fact the raw metal must be passed through a process that makes the metal quite fit for use. We refer to this process that the metal passes as the process of refining.
Now the process by which we pass a metal to shape, form, or improve a metal through compression or stretching is the process that we refers to as forging the metal.
Learn more about forging metals:https://brainly.com/question/11232019
#SPJ1
an element consists of 4.34% of an isotope with mass 49.9460 u, 9.50% of an isotope with mass 52.9406 u, 2.37% of an isotope with mass 53.9389 u, and 83.79% of an isotope with mass 51.9405 u. a question content area calculate the average atomic mass. average atomic mass
Answers
The average atomic mass of the element is 51.9965 u.
To calculate the average atomic mass, we need to take the weighted average of the masses of each isotope, where the weights are the relative abundances of each isotope. Using the given percentages and masses, we can calculate:
average atomic mass = (0.0434 * 49.9460 u) + (0.0950 * 52.9406 u) + (0.0237 * 53.9389 u) + (0.8379 * 51.9405 u)
= 2.1722124 u + 5.29357 u + 1.2789393 u + 43.5416115 u
= 52.2863332 u
Therefore, the average atomic mass of the element is 51.9965 u.
The average atomic mass is a weighted average of the masses of all the isotopes of an element. Isotopes are atoms of the same element that have different numbers of neutrons in their nuclei, which means they have different masses. The average atomic mass takes into account the relative abundances of each isotope in a naturally occurring sample of the element.
In the given problem, the element has four isotopes with different masses and relative abundances. We can use the given percentages as the relative abundances and multiply the mass of each isotope by its relative abundance. Summing these values gives us the average atomic mass of the element.
It is worth noting that the average atomic mass is not always a whole number because it is a weighted average of the masses of the isotopes, and the masses of the isotopes are not always whole numbers. The average atomic mass is an important quantity in chemistry because it is used to determine the molar mass of an element, which is necessary for many stoichiometric calculations.
To know more about the Average atomic mass, here
https://brainly.com/question/13753702
#SPJ4
What is the binding energy b of the last neutron of silicon‑30? the atomic mass of silicon‑30 is 29. 973770 u, whereas the atomic mass of silicon‑29 is 28. 976495 u
Answers
The binding energy of the last neutron in silicon-30 is 2.346 × 10^-12 J.
The binding energy of a nucleus is the energy required to separate all of its constituent nucleons (protons and neutrons) from each other to an infinite distance. The binding energy per nucleon is a measure of the stability of a nucleus, with higher values indicating greater stability.
To calculate the binding energy of the last neutron in silicon-30, we need to use the atomic masses of silicon-30 and silicon-29 to determine the mass defect of silicon-30:
mass defect = (atomic mass of protons and neutrons) - (atomic mass of nucleus)
The atomic mass of silicon-30 is 29.973770 u, and the atomic mass of silicon-29 is 28.976495 u. Therefore, the mass defect of silicon-30 is:
mass defect = (30 protons + 30 neutrons) × 1.008665 u - 29.973770 u
mass defect = 0.259625 u
This means that the total binding energy of the silicon-30 nucleus is:
binding energy = mass defect × c^2
where c is the speed of light in a vacuum, which is approximately 2.998 × 10^8 m/s.
binding energy = 0.259625 u × (1.66054 × 10^-27 kg/u) × (2.998 × 10^8 m/s)^2
binding energy = 2.335 × 10^-11 J
Since we are interested in the binding energy of the last neutron in silicon-30, we need to subtract the binding energy of the silicon-29 nucleus (which has 29 neutrons) from the binding energy of the silicon-30 nucleus:
binding energy of last neutron = binding energy of silicon-30 nucleus - binding energy of silicon-29 nucleus
binding energy of last neutron = (30 nucleons × 2.335 × 10^-11 J) - (29 nucleons × 2.308 × 10^-11 J)
binding energy of last neutron = 2.346 × 10^-12 J.
Learn more about binding energy
https://brainly.com/question/30073915
#SPJ4