a typical bagel from the 1970s or 80s was 3 inches in diameter and provided about 140 calories. about how many calories does a typical bagel today provide?
Answers
A typical bagel today is larger than the ones from the 1970s and 80s, with an average diameter of around 4-6 inches. Due to this increase in size, a modern bagel provides about 250-300 calories.
The larger size contributes to the higher caloric content, but the ingredients used in bagels have remained relatively consistent. Bagels are commonly made from wheat flour, water, yeast, and salt, with added sweeteners such as malt or sugar. They are then boiled before being baked, giving them their unique texture.
Bagel consumption has also expanded beyond traditional flavors like plain, sesame, or poppy seed. Today, you can find a wide variety of bagel flavors and toppings, which can further increase their caloric content. Additionally, bagels are often served with spreads or fillings like cream cheese, butter, or deli meats, adding even more calories to your meal.
In summary, a typical bagel today provides about 250-300 calories, mainly due to the increased size compared to bagels from the 1970s and 80s.
To learn more about caloric content click here
brainly.com/question/31321370
#SPJ11
To determine how many calories a typical bagel today provides, we need to know the diameter of a typical modern bagel and compare it to the 1970s or 80s bagel. Since the diameter of a bagel from the 1970s or 80s was 3 inches and provided about 140 calories, we can use this information to find the caloric density per square inch and then apply it to the modern bagel.
Step 1: Find the area of the 1970s or 80s bagel.
Area = π(radius)^2
Area = π(1.5)^2
Area ≈ 7.07 square inches
Step 2: Find the caloric density per square inch.
Caloric density = 140 calories / 7.07 square inches ≈ 19.8 calories/square inch
Step 3: Find the diameter of a typical modern bagel.
Unfortunately, the diameter of a typical modern bagel is not provided in the question, so we cannot accurately calculate the number of calories it provides. If you can provide the diameter of a typical modern bagel, we can complete the calculation.
To know more about caloric density:
https://brainly.com/question/18891496
#SPJ11
Related Questions
When a metal such as copper is heated,it expands. Can anyone tell me what happens to the metal particles as the solid metal expands?
Answers
Answer:
The metal molecules inside start to vibrate faster, causing the solid to change to liquid if exposed to the intense temperature over a long period of time.
Explanation:
As my answer states, but for an explanation, lets say you are boiling an ice cube, it will start to turn into liquid water, that is because it has been exposed to that heat for a long period of time. The same will happen for the metal, if its exposed for long enough, it will turn into a liquid, then can be turned into a gas because the molecules are vibrating so quickly that they start to easily move apart.
Hope this helps, let me know if any more clarification is needed.
1: An unsaturated hydrocarbon B upon treatment with Hydrogen bromide produces compound C. Compound C reacts with sodium metal in the presence of organic ether produces compound D of molecular formulae C6H14
i• Give the chemical equations for the conversion of compound B to compound C and compound D.
ii• Write down the IUPAC name of compound C and D.
iii• Give the structural formulae of positional isomer of compound C.
Answers
The chemical equations, IUPAC name, and Structural formulas are given below.
i. Chemical equations:
a) Conversion of unsaturated compound B to compound C:
B + HBr → C (Addition of hydrogen bromide to unsaturated B to form bromohexane C)
b) Conversion of compound C to compound D:
C + Na + Ether → D (Reaction of bromohexane C with sodium metal in the presence of ether to form compound D)
ii. IUPAC names:
Compound C: Bromohexane
Compound D: Hexane
iii. Structural formulae of positional isomers of compound C:
Positional isomers of bromohexane can have different bromine atoms attached at different positions along the hexane chain. Here is an example of one positional isomer of bromohexane:
1-Bromohexane:
CH3CH2CH2CH2CH2CH2Br
2-Bromohexane:
CH3CH2CH2CH2CH2CHBr
3-Bromohexane:
CH3CH2CH2CH2CHBrCH3
Therefore, the chemical equations, IUPAC name, and Structural formulas are provided above.
Learn more about IUPAC name here:
https://brainly.com/question/11516594
#SPJ1
Describe the change that you made that led to the increase in the size of the clawcat population. Explain why the change led to an increase in the clawcat population size.
Answers
The first step is to lessen the number of the organism which consumes clawcats. The second is to enhance the resources Clawncat requires to live.
What is population?Population is the total number of people living in a region (such as a nation or the planet), which is always changing due to births, immigration, and natural mortality.
There are two ways to boost the Clawcat population. The first step is to lessen the number of the organism which consumes clawcats. The second is to enhance the resources Clawncat requires to live, namely by expanding the plantation of Clawncat, providing enough irrigation, giving full access to sunshine, and increasing the richness of the soil through proper and effective fertilization.
Therefore, in the above given ways we can increase the population of Clawncat.
To learn more about population, here:
https://brainly.com/question/27779235
#SPJ1
Ya brainly no SIRVEEEER ☹
Answers
Answer:
???? Hmmmmmmkkkkkkkk
Answer:
brainly brainly brainly
pls cange
Explanation:
Give the o.N. Of each of the elements magnesium and oxygen in the reactants and in the products 2Mg + O2=2MgO
Answers
Answer:
\(2Mg^0 + O_2^0\rightarrow2Mg^{2+}O^{2-}\)
Explanation:
Hello there!
In this case, according to the rules for the oxidation states in chemical reactions, it is possible to realize that lone elements have 0 and since magnesium is in group 2A, it forms the cation Mg⁺² as it loses electrons and oxygen is in group 6A so it forms the anion O⁻²; therefore resulting oxidation numbers are:
\(2Mg^0 + O_2^0\rightarrow2Mg^{2+}O^{2-}\)
Best regards!
Which of the following reagents would oxidize Zn to Zn²⁺, but not Pb to Pb²⁺?
Ca²⁺
Co
Br₂
Br⁻
Ca
Co²⁺
Answers
The reagent that would oxidize Zn to Zn²⁺ but not Pb to Pb²⁺ is CoBr₂.
To determine the reagent that selectively oxidizes Zn to Zn²⁺ without oxidizing Pb to Pb²⁺, we need to consider the reduction potentials of the species involved.
The reagent with a higher reduction potential will oxidize the species with a lower reduction potential.
In this case, Zn has a lower reduction potential than Pb, which means it is easier to oxidize Zn to Zn²⁺ compared to Pb to Pb²⁺. Among the given options, CoBr₂ is the only reagent that can selectively oxidize Zn to Zn²⁺ without oxidizing Pb.
Therefore, the correct answer is CoBr₂. It has a higher oxidizing power and can oxidize Zn to Zn²⁺ while leaving Pb unaffected.
Learn more about reagent here: brainly.com/question/28463799
#SPJ11
calculate the percent of lys that has its side chain deprotonated at ph 7.4.
Answers
To calculate the percentage of lysine (Lys) side chains that are deprotonated at pH 7.4, we need to consider the pKa of the lysine side chain and the pH of the solution.
The side chain of lysine contains an amino group (NH2) with a pKa value of approximately 10.5. At a pH below the pKa, the amino group is mostly protonated (NH3+), while at a pH above the pKa, the amino group is mostly deprotonated (NH2).
Given that the pH is 7.4 (which is below the pKa of lysine), we can assume that the amino group is mostly protonated.
Therefore, at pH 7.4, the percentage of lysine side chains that are deprotonated (NH2) is negligible.
Hence, the percent of lysine side chains that have their side chain deprotonated at pH 7.4 is close to 0%.
To know more about lysine refer here
brainly.com/question/32240062#
#SPJ11
Which answer best describes what is happening in the following redox reaction?
4Fe + 302.
2Fe2O3
Answers
Answer:
Iron is oxidized to form rust.
Explanation:
Consider the reaction; 4Fe + 302------>2Fe2O3, we can see that iron is being oxidized to iron III oxide. Rust is the common name of iron III oxide.
Rusting is an electrochemical process, iron rusts when it comes into contact with air and water because electrochemical cells are set up at the surface of contact.
Iron usually functions as the anode in the electrochemical process. This process leads to the formation of iron III oxide. Rust is soft and breaks off easily thereby exposing the metal below the surface to further rusting.
Answer:
Iron is oxidized to form rust.
Explanation:
edge
b. 8500°C 600°C d. 3700°C с C 9. Which organic acid is found in apple a. Tannic acid b. Malic acid c) Lactic acid d citric acid
Answers
Answer:
b. Malic Acid
Explanation:
Malic (organic) Acid is found in apple.
GL
Question:
In a laboratory demonstration, a balloon filled with methane and oxygen was exposed to a
flame. The result was a brief, large flame. The students were asked to formulate an equation for
the reaction. One answer is below.
CH, + 0 = CO,
This equation is incorrect.
A. Explain how and why it is incorrect
B. What would the correct equation be, and how do you know that?

Answers
Answer:
The laboratory demonstration consists of the following;
The compounds present in the combustion reaction = Methane, CH₄ and Oxygen, O₂
The chemical equation for the combustion reaction is given as follows;
CH₄ + 2O₂ → CO₂ + 2H₂O
Therefore;
A. The equation given as CH₄ + O → CO₂ is not correct because;
1) Oxygen gas exist as diatomic molecules, O₂, and given that the experiment involves the mixture of gases, the oxygen gas present which can exist as a separate compound, should be represented as O₂
2) The number of oxygen molecules in the reaction is two rather than one
3) The product also includes two molecules of water (vapor) H₂O
B. The correct equation for the reaction should be given as follows;
CH₄ + 2O₂ → CO₂ + 2H₂O
B i) The constituents of the equation is obtained by the knowledge of the fact that the combustion reaction of an organic substance such as methane in the presence of oxygen yields, carbon dioxide and water (vapor)
The equation showing the relative amounts the reacting compounds is by balancing the basic equation of the combustion of methane in the presence of oxygen
Explanation:
According to the article, which of the following organisms were NOT impacted by the rabbits?
A cattle
B salt water crocodile
C greater bilby
D sheep
Answers
Answer:
B salt water crocodile
Explanation:
took test and this was right :)

why is edta used to determine the hardness of water
Answers
EDTA (ethylenediaminetetraacetic acid) is commonly used to determine the hardness of water due to its ability to form complexes with metal ions, particularly calcium and magnesium ions.
Water hardness refers to the concentration of calcium and magnesium ions present in water. These ions can cause scaling, reduce the effectiveness of soaps, and have other negative effects. EDTA acts as a chelating agent, meaning it can bind to metal ions and form stable complexes.
In the process of determining water hardness, a known amount of EDTA solution is added to a water sample. The EDTA molecules form complexes with the calcium and magnesium ions present in the water.
The endpoint of the titration is reached when all the metal ions are complexed by the EDTA, resulting in a color change or an indicator reaching a specific endpoint.
To know more about hardness of water refer:
https://brainly.com/question/28178305
#SPJ11
Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 ml, buffer containing 0.13 M HCIO and 0.37 M NaCIO. The value of Ka for HCIO is 2.9 x 10^-4
Answers
The resulting pH of the buffer after 0.003 mol of solid NaOH is added is 4.81.
To determine the resulting pH when 0.003 mol of solid NaOH is added to the buffer solution, we need to first calculate the initial pH of the buffer before the NaOH is added.
Using the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
where pKa = -log(Ka) and [A-]/[HA] is the ratio of the concentrations of the conjugate base (A-) and acid (HA) components of the buffer.
First, we need to calculate the concentrations of HCIO and CIO- in the buffer:
[HClO] = 0.13 M
[ClO-] = 0.37 M
Next, we need to calculate the ratio of [ClO-]/[HClO]:
[ClO-]/[HClO] = (0.37 M)/(0.13 M) = 2.846
Now, we can calculate the pKa:
pKa = -log(2.9 x 10^-4) = 3.54
Using the Henderson-Hasselbalch equation, we can calculate the initial pH of the buffer:
pH = 3.54 + log(2.846) = 4.62
So, the initial pH of the buffer is 4.62.
Next, we need to determine how the addition of 0.003 mol of solid NaOH affects the buffer. The NaOH will react with the HCIO in the buffer to form water and the conjugate base CIO-:
NaOH + HClO → NaClO + H2O
The amount of HCIO that reacts with the NaOH is equal to the amount of NaOH added, since the reaction is a 1:1 stoichiometry:
moles of HCIO reacted = 0.003 mol
This means that the concentration of HCIO in the buffer is now:
[HClO] = (0.13 M)(100.0 mL)/(100.0 mL) = 0.13 M
And the concentration of CIO- is:
[ClO-] = (0.37 M)(100.0 mL)/(100.0 mL) + (0.003 mol)/(100.0 mL) = 0.373 M
Using the new concentrations of HCIO and CIO- in the Henderson-Hasselbalch equation, we can calculate the new pH of the buffer after the NaOH is added:
pH = pKa + log([ClO-]/[HClO])
pH = 3.54 + log(0.373/0.13)
pH = 4.81
Learn More pH about here :-
https://brainly.com/question/30656928
#SPJ11
I’ve tried to do the problem but it’s confusing

Answers
Answer:
Explanation:
The equation for calculating the amount of heat released is:
q = mcΔT
where:
q = heat released (in joules)
m = mass of the substance (in grams)
c = specific heat capacity (in J/g·°C)
ΔT = change in temperature (in degrees Celsius)
Given:
m = 30 g
ΔT = 96°C - 25°C = 71°C
c = 4.184 J/g·°C (for water)
so
q = (30 g)(4.184 J/g·°C)(71°C) = 8.91*71 = 635.11 J
Therefore, 635.11 J of heat is released when 30g of water cools down from 96 degree celsius to 25 degree celsius
Note that this calculation is valid only if the process is adiabatic or no heat is exchanged with the environment.
write a
paragraph or two on electromagnetism.
Answers
Answer:
Electromagnetism is the study of the electromagnetic force, one of the four fundamental forces of nature. It includes the electric force, which pushes all charged particles, and the magnetic force, which only pushes moving charges.It is used in many electrical appliances to generate desired magnetic fields. It is even used in a electric generator to produce magnetic fields for electromagnetic induction to occur.
Explanation:
tell me if this helped, ill try and explain better
this is a tiny unit of an element that retains the properties of the element.
Answers
The tiny unit of an element that retains the properties of the element is called an atom.
Atoms are the basic building blocks of matter and are composed of a nucleus, which contains positively charged protons and neutral neutrons, surrounded by negatively charged electrons that orbit the nucleus. The unique arrangement of protons, neutrons, and electrons in an atom determines its chemical and physical properties, such as its atomic number, mass, and reactivity.Atoms are incredibly small and are typically measured in units of picometers or angstroms.
Each element is defined by the number of protons in its atomic nucleus, which is known as its atomic number. For example, carbon atoms have six protons in their nucleus, while oxygen atoms have eight protons. The number of neutrons in an atom's nucleus can vary, resulting in isotopes of the same element with different atomic masses.
Atoms can combine with other atoms to form molecules and compounds through chemical bonds, including covalent bonds, ionic bonds, and metallic bonds.
Learn more about atoms here:
https://brainly.com/question/13654549
#SPJ4
The steroid molecule below is a metabolic precursor of both testosterone and estrone. What functional group(s) are present? A) ketone CH3 B) aldehyde ether D) ester E) ether and ester CH3'
Answers
The functional group(s) present in the steroid molecule are:
A) Ketone (CH3)
D) Ester (CH3')
The steroid molecule contains a ketone functional group (CH3) and an ester functional group (CH3').
What are steroids?
Steroid molecules are a class of organic compounds characterized by a specific four-ring structure consisting of three cyclohexane rings and one cyclopentane ring fused together. Steroids are derived from cholesterol and serve as important hormones and signaling molecules in the body. Examples of steroid molecules include testosterone, estrogen, progesterone, and cortisol.
To know more about steroids, refer here:
https://brainly.com/question/928165
#SPJ4
HELP ASAP ONLY HAVE FEW MINUTES!
Help with both please not only one .

Answers
Answer:
For the first one I think it is:
2)Cell 5)tissue 3)organ 1)organism 4)body/organ system
I also think the second question is B
what is the chemical formulae for lime water
Answers
Answer:
The formula for lime water is Ca(OH)2 and the chemical name for lime water is calcium hydroxide.Explanation:
#Let's Study
#I Hope It's Help
#Keep On Learning
#Carry On Learning
\(\\ \purple{MaggieEve}\)
The hydrogen gas produced is collected over water at 25.0 oC. The volume of the gas is 7.80 L, and the total pressure in the gas collection vessel is 0.980 atm. Calculate the mass of zinc metal that reacted in grams.
Answers
Answer:
20.4 grams Zn
Explanation:
To find the mass, you first need to find the moles. This can be found using the Ideal Gas Law equation:
PV = nRT
In this equation,
-----> P = pressure (atm)
-----> V = volume (L)
-----> n = moles
-----> R = Ideal Gas Constant (0.08206 atm*L/mol*K)
-----> T = temperature (K)
Before you can plug the values into the equation, you need to convert Celsius to Kelvin.
P = 0.980 atm R = 0.08206 atm*L/mol*K
V = 7.80 L T = 25.0 °C + 273.15 = 298.15 K
n = ? moles
PV = nRT
(0.980 atm)(7.80 L) = n(0.08206 atm*L/mol*K)(298.15 K)
7.644 = n(24.466)
0.312 moles = n
Now that you have the number of moles, you can convert it to grams using the atomic mass of zinc. The final answer should have 3 sig figs to match the sig figs in the given values.
Atomic Mass (Zn): 65.380 g/mol
0.312 moles Zn 65.380 grams
------------------------- x ------------------------- = 20.4 grams Zn
1 mole
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
Which is more dense, dish soap or corn syrup?
Answers
Answer:
Corn syrup has 1.33 and dish soap has 1.06 so its corn syrup
What volume will be occupied by 40.2 g of neon gas at STP?
Answers
Answer:
44.8 L
Explanation:
1 mole of a gas at s.t.p. occupies a volume of 22.4 L.
Molar mass of Neon is 20.1 g/mol
Number of moles of neon present in 40.2 g = Mass/molar mass
Number of moles = 40.2 / 20.1 = 2 moles.
Therefore, 2 moles of neon gas will occupy 2 * 22.4 L = 44.8 L
44.8 L
Molar mass of Neon is 20.1 g/mol
Number of moles of neon present in 40.2 g =\(Mass/molar mass\)
\(Number of moles = 40.2 / 20.1 \\= 2 moles.\)
At STP:-
Gases have a volume of 22.4L/mol.
Therefore, 2 moles of neon gas will occupy the volume is as follows:-
\(2 mol\times22.4 L/mol = 44.8 L\)
So, the volume is 44.8 L.
To know more about:-
brainly.com/question/24050436
I would like to see some of your answers given these equations
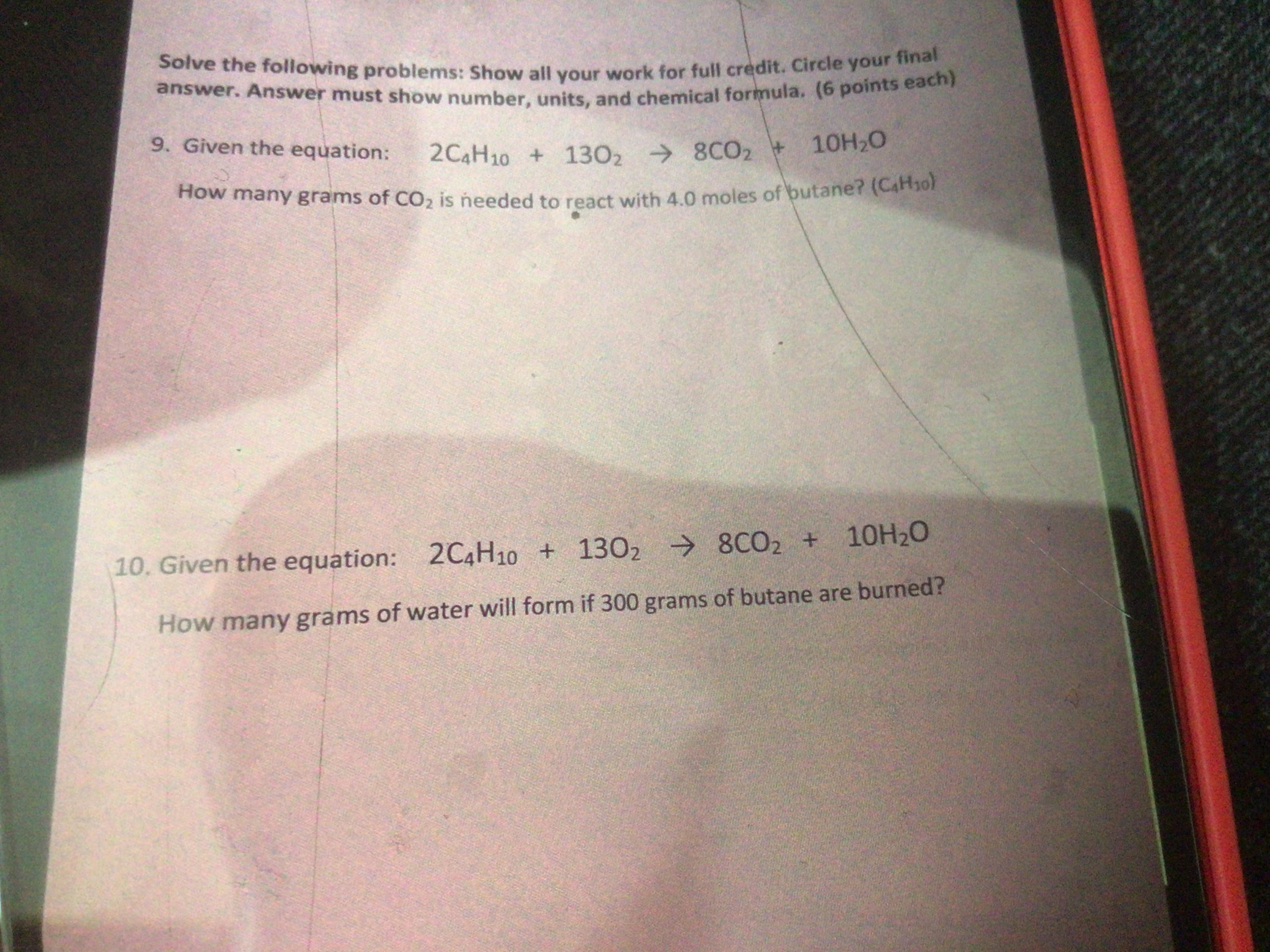
Answers
2.816 g of carbon dioxide is needed to react with 4 moles of butane in the reaction 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O.
Those reaction in which fuel is oxidized by the oxygen molecules and produce carbon dioxide and water molecule.
Given chemical reaction is :
2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
Given mass of water = 2.46 grams
Moles will be calculated as:
n = W/M,
where
W = given mass
M = molar mass
Moles of water formed is calculated as:
Moles of water n = 2.46g / 18 g/mol = 0.137moles
From the stoichiometry of the reaction, it is clear that:
10 moles of water = produced by 2 moles of butane
0.137 moles of water = produced by 2/10×0.137 = 0.0274 moles of butane
Weight of butane is calculated by using moles:
W = 0.0274 × 58 g/mol = 1.5892 g
From the stoichiometry of the reaction, it is clear that:
2 moles of butane = react with 13 moles of O₂
4 moles of butane = react with 13/4 ×0.027 = 0.088 moles of O₂
Mass of oxygen is calculated as:
W = 0.088 x 32 g/mol = 2.816 g
To know more about combustion reaction here
https://brainly.com/question/3122366
#SPJ1
The quality of a two-phase liquid–vapor mixture of h2o at 40°c with a specific volume of 10 m3/kg is.
Answers
The quality of the two-phase liquid-vapor mixture of H2O at 40°C with a specific volume of 10 m3/kg is approximately 0.1176 or 11.76%.
The quality of a two-phase liquid-vapor mixture is the fraction of the total mass that is in the vapor phase. The specific volume of a substance is the volume occupied by one kilogram of that substance.
Since the mixture is two-phase, it means it is a combination of liquid and vapor phases. At a given temperature and pressure, the quality of a mixture is determined by its specific volume.
Given:
Temperature of mixture (T) = 40°C
Specific volume of mixture (v) = 10 m3/kg
Using the saturated water table, we can find that at 40°C, the specific volume of the saturated liquid (vf) is 0.001067 m3/kg and the specific volume of the saturated vapor (vg) is 0.08608 m3/kg.
Since the mixture is two-phase, we can use the following equation to calculate the quality:
x = (v - vf)/(vg - vf)
where x is the quality of the mixture.
Plugging in the values, we get:
x = (10 - 0.001067)/(0.08608 - 0.001067)
x = 0.1176
To know more about specific volume refer to-
https://brainly.com/question/1463418
#SPJ11
in which compound does oxygen have an oxidation number other than −2? select the correct answer below: co2 h2o h3po4 h2o2
Answers
Out of the compounds listed below, the compound in which oxygen has an oxidation number other than −2 is H₂O₂. The correct option is (d)H₂O₂.
Oxidation number can be described as the number that is given to an atom of an element when it combines with other atoms. It is assigned to an atom of an element in a particular compound, which shows its ability to either donate or accept electrons when it reacts with other atoms.
The oxidation number of an atom in a molecule indicates the electron sharing that occurs in chemical bonds. The general rules for determining the oxidation number of an atom are: In an uncombined or elemental state, atoms have an oxidation number of 0.
Ions' oxidation numbers are the same as their charges. Oxygen in most of the compounds has an oxidation state of -2 except in peroxides, where it has an oxidation state of -1. In H₂O₂, the oxygen atoms have an oxidation number of -1. Hence, H₂O₂ is the only compound from the given options where oxygen has an oxidation number other than −2. Hence, d is the correct option.
You can learn more about oxidation numbers at: brainly.com/question/29100691
#SPJ11
a chemical ____ is a process in which some substances change chemically into different substances
Answers
Answer:
Explanation:
a chemical reaction is a process in which the some substances go into reactant and some into products , the reactant molecule produce the new molecules in the form of product, it's also called the breaking of chemical bonds and forming the new bonds .
A chemical property is a change in _____.
hardness
density
composition of matter
physical state
Answers
Answer:
Composition of matter
3. A substance which is made up of two are more different
elements chemically combined is known as *
A. an atom
B. a substance
C. a compound
D. a molecule
Answers
D. Molecules
The a helix of the secondary structure of a protein is held together by between two widely separated parts of a proteirn chain. Select one: O a. hydrophilic interactions O b. hydrogen bonds O c. hydrophobic interactions O d. disulfide bridges O e. salt bridges
Answers
A hydrogen bond (b) is a weak force of attraction that occurs between a hydrogen atom covalently bonded to an electronegative atom and another electronegative atom or group of atoms.
The a helix of the secondary structure of a protein is held together by hydrogen bonds between two widely separated parts of a protein chain.
Hydrogen bonds are weak bonds that hold the backbone N-H groups and C=O groups of neighboring amino acids close together in an α-helix structure. These bonds are formed between the oxygen atom in the C=O group of one amino acid and the hydrogen atom in the N-H group of another amino acid.
In an α-helix, the hydrogen bonds occur between the carboxyl oxygen atom of one amino acid and the amide hydrogen atom of the fourth amino acid away from it in the sequence.
Thus, the a helix of the secondary structure of a protein is held together by hydrogen bonds between two widely separated parts of a protein chain.
A hydrogen bond is a special type of electrostatic attraction between a hydrogen atom, covalently bonded to a more electronegative atom, and another electronegative atom or group of atoms. A hydrogen bond is a weak force of attraction that occurs between a hydrogen atom covalently bonded to an electronegative atom and another electronegative atom or group of atoms.
To know more about electronegative visit:
https://brainly.com/question/29597673
#SPJ11