A student sets a 0. 4 kilogram (kg) soccer ball on the ground and gives it a hard kick. The ball traveled a distance of 37 meters (m). The acceleration of the ball was 2,550 meters per second squared (m/s). Based on the data, how much force in newtons (N) did the student apply to the ball?
A
Answers
Nased on the mentioned informations, the student is calculated to have applied a force of 1,020 newtons to the soccer ball.
To calculate the force applied by the student to the ball, we can use the formula:
Force = mass x acceleration
We are given the mass of the soccer ball, which is 0.4 kg, and the acceleration of the ball, which is 2,550 m/s².
So, substituting the values in the formula, we get:
Force = 0.4 kg x 2,550 m/s²
Force = 1,020 N
Therefore, the student applied a force of 1,020 newtons to the soccer ball.
Learn more about force :
https://brainly.com/question/19045196
#SPJ4
Related Questions
How much of a sample remains after five half-lives have occurred?
1/5 of the original sample
1/25 of the original sample
1/32 of the original gample
1/64 of the original sample
Answers
The first half-life, we have 1 • 1/2 = 1/2 left.
After two: 1/2 • 1/2 = 1/4
Three: 1/4 • 1/2 = 1/8
Four: 1/8 • 1/2 = 1/16
And five: 1/16 • 1/2 = 1/32.
Answer:
1/32
Explanation:
the molar heat capacity of silver is 25.35 j/mol⋅∘c . how much energy would it take to raise the temperature of 8.20 g of silver by 17.3 ∘c ? express your answer with the appropriate units.
Answers
33.34 J of energy it would take to raise the temperature.
Specific heat = J/g C
(25.35 J/mole C) x (1 mole / 107.8682g) = 0.2350 J/g C
Energy required = (mass in grams)x(Specific heat)x(Temp final - Temp initial)
= (8.20 g)(0.2350 J/g C)(17.3°C) = 33.34 J (units cancel for /g C)
Heat capacity or specific heat is the quantity of heat consistent with unit mass that is required to elevate the temperature with the aid of 1°C. particular warmness is helpful in figuring out the processing temperatures and quantity of warmth vital for processing and may be helpful in differentiating between polymeric composites.
The specific heat capacity (or in reality, the specific heat), that's the heat potential according to unit mass of a material. Experiments show that the transferred heat relies upon on three factors: (1) The change in temperature, (2) the mass of the system, and (3) the substance and phase of the substance.
The specific heat is the quantity of heat energy per unit mass required to elevate the temperature by way of one degree Celsius. The connection between warmth and temperature change is commonly expressed inside the form shown under where c is the unique warmness .
Learn more about Specific heat here:- https://brainly.com/question/21406849
#SPJ1
A sample of gas at 240K and 670 mmHg occupies a 1.28L volume. What volume (in Liters) will the gas occupy at 198K if the pressure is changed to 680 mmHg?
Answers
Answer:
1.04 L.
Explanation:
What is given?
Temperature 1 (T1) = 240 K.
Pressure 1 (P1) = 670 mmHg.
Volume 1 (V1) = 1.28 L.
Temperature 2 (T2) = 198 K.
Pressure 2 (P2) = 680 mmHg.
What do we need? Volume 2 (V2).
Step-by-step solution:
To solve this problem, we have to use the combined gas law. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. For a combined gas law problem, only the amount of gas is held constant. The formula of combined gas law is:
\(\frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2}.\)We need to find the volume 2 (V2), so let's solve for this unknown value and let's replace the given data that we have:
\(\begin{gathered} V_2=\frac{P_1V_1T_2}{T_1P_2}, \\ V_2=\frac{670\text{ mmHg}\cdot1.28\text{ L}\cdot198K}{240K\cdot680\text{ }mmHg}, \\ V_2=1.04\text{ L.} \end{gathered}\)The final volume for this case would be 1.04 L, the volume is being reduced.
Describe what you should do to recover only the water from a sample of muddy water.
Answers
Answer:
By using a filter.
Explanation:
If 0. 735J of heat is added to 0. 9916g of water, how much will the temperature increase?
Answers
The temperature of the water will increase by 0.177°C when 0.735 J of heat is added to 0.9916 g of water.
The specific heat capacity of water is 4.184 J/g °C, which means that 4.184 J of heat is needed to raise the temperature of 1 g of water by 1°C. The amount of heat required to raise the temperature of a substance is determined by its mass and specific heat capacity. The formula is:
Q = mcΔT
Where:
Q = Heat capacity
m = Mass of the substance
c = Specific heat capacity of the substance
ΔT = Change in temperature
To calculate the change in temperature when 0.735 J of heat is added to 0.9916 g of water, the formula can be rearranged as:
ΔT = Q / (mc)
ΔT = 0.735 J / (0.9916 g × 4.184 J/g °C)
ΔT = 0.735 J / (4.1486 J/g°C × g)
ΔT = 0.177°C (rounded to three significant figures)
Therefore, the temperature of the water will increase by 0.177°C when 0.735 J of heat is added to 0.9916 g of water.
Learn more about temperature
https://brainly.com/question/15267055
#SPJ11
2. The energy of a photon that has a wavelength of 12.3 nm is _______ J.
A) 2.72 x 10^-50
B) 1.62 x 10^-17
C) 4.42 x 10^-23
D) 1.99 x 10^-25
E) 1.51 x 10^-17
Answers
Classify the following compounds as weak acids (W) or strong acids (S): hydrocyanic hydrofluroic phenol
Answers
Hydrocyanic and hydrofluoric are both weak acids (W), while phenol is a strong acid (S).
Acids are substances in water that can be ionized to release hydrogen ions or hydronium ions. While a base is a substance in water that can be ionized releasing hydroxide ions.
This classification is based on the strength of their conjugate bases. Hydrocyanic acid (HCN) and hydrofluoric acid (HF) have weakly basic conjugate bases (CN⁻and F⁻), meaning they do not readily donate a proton (H⁺) to water molecules. Phenol (C₆H₅OH), on the other hand, has a strongly basic conjugate base (C₆H₅O⁻), meaning it readily donates a proton to water molecules, making it a strong acid.
Learn more about weak acids at https://brainly.com/question/24586675
#SPJ11
3.25 kcal is the same amount of energy as A. 3.25J. B. 0.7771. C. 777J. D. 13600 j.
Answers
Kilocalorie is a unit of measuring the amount of energy of a reaction, but this is not the only unit, we can also have Joules as a unit, and the conversion is:
1 Kcal = 4184 Joules
Therefore if we have 3.25 Kcal, we will have:
3.25 * 4184 = 13600 Joules of energy, therefore letter D
Hydrogen peroxide decomposes back to water and oxygen when exposed to air and light: When bought at a pharmacy for home use, hydrogen peroxide is sold in dark bottles that are labeled as concentration of 3% by weight A chemistry student wants t0 test the concentration of hydrogen peroxide that has been decanted to light and dark bottles; and exposed to air for 10, 20 and 30 hours The concentration is tested by titration with potassium permanganate; and is tested twice at each combination of levels. response variable(s) concentration explanatory variablels) or factor(s) color; hours experimental unitls) dark; light number of treatment(s)
Answers
The concentration is tested by titration with potassium permanganate, and each sample is tested twice Replication- 10/20/30 hours.
Hydrogen peroxide is a chemical compound that is gaseous in nature.
Since it is unstable it slowly decomposes in the presence of light to form water and oxygen. The control experiment is necessary for the determination of reliability and statistical significance of results obtained.
Testing the concentration of the species after exposure to air for 10,20 and 30 hours is randomization. Replication refers to the number of times the same experiment is carried out. It enhances the reliability of the result of an experiment.
Testing each sample twice is replication.
To know more about the Hydrogen peroxide, here
brainly.com/question/18709693
#SPJ4
What mass of HI should be present in 0.200L of solution to obtain a solution with each of the following pH's?
pH=1.20
pH=1.75
pH=2.85
Answers
The mass of HI should be present in 0.200l of solution to obtain a solution with pH value's,
(a) pH value is 1.20 the mass is 1.08g
(b) pH value is 1.75 the mass is 0.0066g
(c) pH value is 2.85 the mass is 0.00012g
To solve this problem, we must determine the concentration of H+ ions in the solution using the pH of the solution and the dissociation constant of HI. The concentration of HI and the mass of HI required to make the solution may then be calculated.
The dissociation reaction for HI is:
HI(aq) ↔ H+(aq) + I-(aq)
The dissociation constant, Ka, for this reaction, is:
Ka = [H+][I-]/[HI]
This formula may be simplified by assuming that the starting concentration of HI is equal to the concentration of I- produced, which is equal to the concentration of H+ produced due to the reaction's 1:1 stoichiometry. This results in:
Ka = [H+]^2/[HI]
Solving for [H+], we get:
[H+] = sqrt(Ka*[HI])
Taking the negative log of both sides gives us the pH of the solution:
pH = -log[H+] = -log(sqrt(Ka*[HI]))
pH= -0.5*log(Ka) - 0.5*log([HI])
Rearranging this equation, we get:
[HI] = 10^(-(pH + 0.5*log(Ka)))/V
where V is the volume of the solution.
Now we can calculate the mass of HI required for each pH:
(a) For pH = 1.20:
Ka for HI is 1.3 x 10^-10. Substituting this value into the equation above, we get:
[HI] = 10^(-(1.20 + 0.5*log(1.3 x 10^-10)))/0.200L ≈ 0.0042 M
The mass of HI required is:
mass = concentration x volume x molar mass
= 0.0042 mol/L x 0.200 L x 127.91 g/mol
≈ 1.08 g
Therefore, approximately 1.08 grams of HI is required to prepare a solution with a pH of 1.20.
(b) For pH = 1.75:
[HI] = 10^(-(1.75 + 0.5*log(1.3 x 10^-10)))/0.200L ≈ 0.00026 M
mass = 0.00026 mol/L x 0.200 L x 127.91 g/mol ≈ 0.0066 g
Therefore, approximately 0.0066 grams of HI is required to prepare a solution with a pH of 1.75.
(c) For pH = 2.85:
[HI] = 10^(-(2.85 + 0.5*log(1.3 x 10^-10)))/0.200L ≈ 0.0000047 M
mass = 0.0000047 mol/L x 0.200 L x 127.91 g/mol ≈ 0.00012 g
Therefore, approximately 0.00012 grams of HI is required to prepare a solution with a pH of 2.85.
Learn more about Dissociation Reaction:
https://brainly.com/question/29411272
#SPJ4
Which shows an isomer of the molecule below?

Answers
The isomer of the molecule in the depicted image would be A.
What are isomers?Isomers are molecules with the same chemical formula but different arrangements of their component atoms. Isomers also have different properties.
The original molecule in the image has a triple bond with 4 carbon atoms. Thus, its isomers must also have a triple bond with 4 carbon atoms.
The only option with a triple bond and 4 carbon atoms in the image is option A.
More on isomers can be found here: https://brainly.com/question/13422357
#SPJ1
What are the chemical and biological indicators of water quality?
Answers
The chemical and biological indicators of water quality are substances that are used to measure the quality of water.
These indicators help to identify the presence of pollutants and other contaminants in water. Some common chemical indicators of water quality include pH, dissolved oxygen, total dissolved solids, and salinity. pH is a measure of the acidity or alkalinity of water, and can indicate the presence of pollutants such as acid rain.
Dissolved oxygen levels can indicate the level of organic matter in the water, while total dissolved solids can indicate the presence of pollutants such as salt. Salinity levels can indicate the presence of pollutants such as chloride.
Biological indicators of water quality include the presence of bacteria, viruses, and other microorganisms. These indicators can help to identify the presence of pathogens that can cause disease in humans and other animals. Some common biological indicators of water quality include fecal coliform bacteria, E. coli, and Giardia.
The presence of these organisms in water can indicate the presence of pollutants such as sewage or animal waste.
Learn more about pH here:
https://brainly.com/question/26856926
#SPJ11
The thermometer in the
paper shows the greatest increase in temperature because black
all wavelengths of visible light. The thermometer in the
paper shows the smallest increase in temperature because white
all colors of visible light. The thermometers in the green, red, and blue paper all show an increase in temperature. However, they don't increase as much as the thermometer in the black paper because these colors
.
Answers
Answer:
The thermometer in the [black] paper shows the greatest increase in temperature because black [absorbs] all wavelengths of visible light. The thermometer in the [white] paper shows the smallest increase in temperature because white [reflects] all colors of visible light. The thermometers in the green, red, and blue paper all show an increase in temperature. However, they don't increase as much as the thermometer in the black paper because these colors [absorb all other colors and reflect their own color.]
Explanation:
Hope this helps you understand!
The thermometer in the black paper shows the greatest increase in temperature because black absorbs all wavelengths of visible light. The thermometer in the white paper shows the smallest increase in temperature because white reflects all colors of visible light. The thermometers in the green, red, and blue paper all show an increase in temperature. However, they don't increase as much as the thermometer in the black paper because these colors absorb all other colors and reflect their own color.
The model of the atom changed as new evidence was discovered. The plum pudding model suggested that the atom was a ball of positive charge with electrons embedded in it. Evidence from the alpha particle scattering experiment led to a change in the model of the atom from the plum pudding model. Explain how. PLS HELP RN :)
Answers
Answer:
so since the model atom was changed as new evidence, and the plum pudding model was a ball of positive charge, the alpha particle scattering which broke up the atom
No one is helping me

Answers
Answer:
displacement =1025km
time=6hours
Explanation:
velocity=displacement /time=1025/6=170.8km/h
what is the binding energy in kj/mol nucleons for silver-109? kj/mol nucleons 47 62 the required masses (g/mol) are:
Answers
The binding energy of silver-109 (Ag-109) in kJ/mol nucleons is not a well-defined concept, as binding energy is typically calculated for atomic nuclei rather than individual isotopes.
The binding energy of an atomic nucleus is the energy required to completely separate all of its constituent protons and neutrons into individual particles. It is usually expressed in units of energy per nucleon, which is the energy required to separate one proton or neutron from the nucleus.
The average binding energy per nucleon for an atomic nucleus is typically highest for medium-mass nuclei, such as those found in the region of the so-called "valley of stability" on the nuclear chart.
The binding energy per nucleon for silver-109 is not likely to be particularly high, as silver is a relatively heavy element and the binding energy per nucleon tends to decrease with increasing atomic number (Z).
Without more information about the specific calculation being used to determine the binding energy of Ag-109, it is not possible to accurately provide a value for the binding energy in kJ/mol nucleons.
The required masses (g/mol) are also not specified in the question, so it is not clear what context these values might be used in.
Learn more about binding energy:
https://brainly.com/question/29756225
#SPJ4
What is the symbol of element Californium?
Answers
Answer:
Californium is a chemical element which is radioactive, its symbol is 'Cf' and atomic number is 98
Answer: the elementale symble is Cf
Explanation: the symble of element is Cf
A sample containing 27. 0 moles of propane gas at a temperature of 25. 0 °C is stored in a 12. 5 liter cylinder. What is the pressure of the gas inside the cylinder?
Answers
The pressure of the gas inside the cylinder is 52.90 atm
Given is the number of moles of gas, the temperature and the volume of the gas and we need to find the pressure of the gas inside the cylinder, for this we can use the ideal gas law equation:
PV = nRT
Where:
P = Pressure of the gas (in units of pressure, such as atm)
V = Volume of the gas (in liters)
n = Number of moles of the gas
R = Ideal gas constant (0.0821 L·atm/(mol·K))
T = Temperature of the gas (in Kelvin)
First, let's convert the temperature from Celsius to Kelvin:
T = 25.0 °C + 273.15 = 298.15 K
Now we can substitute the values into the ideal gas law equation:
P × 12.5 L = 27.0 moles × 0.0821 L·atm/(mol·K) × 298.15 K
Simplifying the equation:
P × 12.5 L = 661.2587 L·atm
Dividing both sides by 12.5 L:
P = 661.2587 L·atm / 12.5 L
P ≈ 52.90 atm
Therefore, the pressure of the gas inside the cylinder is approximately 52.90 atm.
Learn more about ideal gas here:
https://brainly.com/question/15379358
#SPJ1
We can use the ideal gas law equation to determine the pressure of a gas within a cylinder:
PV = nRT
Where:
P is the pressure of the gas (in units of pressure, such as atm)
V is the volume of the gas (in units of volume, such as liters)
n is the number of moles of the gas
R is the ideal gas constant (0.0821 L·atm/(mol·K))
T is the temperature of the gas (in units of temperature, such as Kelvin)
we need to convert the temperature from Celsius to Kelvin:
T(K) = T(°C) + 273.15
T(K) = 25.0 °C + 273.15
T(K) = 298.15 K
Now we can plug the data into the ideal gas law equation as follows:
P * 12.5 L = 27.0 moles * 0.0821 L·atm/(mol·K) * 298.15 K
Simplifying the equation:
P = (27.0 moles * 0.0821 L·atm/(mol·K) * 298.15 K) / 12.5 L
Calculating the pressure:
P ≈ 5.046 atm
As a result, the gas inside the cylinder is under a pressure of about 5.046 atm.
Learn more about Ideal gas law equation, here:
https://brainly.com/question/3778152
#SPJ1
Why does the chance of precipitation increase when the humidity is high?
Because the amount of moisture in the air increases as precipitation falls
Because the amount of moisture in the air decreases as humidity increases
Because the high humidity causes the air to hold more moisture
Because the amount of water vapor in the air increases
Answers
Answer:Because the amount of water vapor in the air increases
Explanation:In humid climates, thunderstorms often cause heavier rain than general wintertime rainfall since moisture content in the air typically is higher in the spring and summer, and since air usually rises at a much more rapid rate within developing thunderstorms than in general winter systems.During very heavy rainfall, where all of the water in the atmosphere is often rained out, the water vapour content governs the amount of rainfall. The higher the humidity the greater the water vapour, and the more rain we're likely to see. HOPE THIS HELPS ^w^
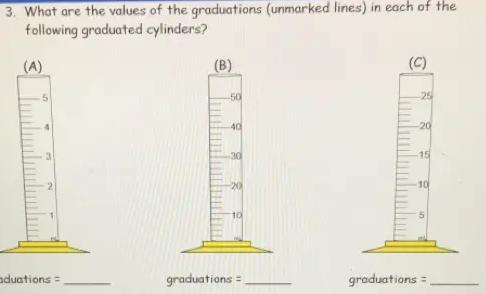
3. What are the values of the graduations (unmarked lines) in each of the
following graduated cylinders?
Q
(A)
50
3
graduations =
(B)
50
-40
-30
-20
10
graduations =
(C)
25
-20
-15
10
5
graduations =
Answers
The values of the Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
Graduated Cylinder - A popular piece of scientific equipment used to measure the volume of a liquid is a graduated cylinder, sometimes referred to as a measuring cylinder or mixing cylinder. Its form is slender and cylindrical. Each marked line on the graduated cylinder indicates the volume of liquid that has been measured.
The clear graduated cylinder is widely used for volume measurements and is regarded to be more accurate than a beaker since it contains permanently marked incremental graduations.
To calculate the value of graduations-
1. Subtract the two values to get the value between the labeled graduations.
2. Determine how many spaces there are between the two graduations. Keep in mind that volume equals space.
3. Divide the number of gaps by the value between the graduations.
For cylinder A.
2 ml- 1ml = 1ml
Number of spaces = 5
∴1/ 5 = 0.2
Graduation = 0.2ml
For cylinder B -
20-10 = 10
number of spaces = 5
∴10/ 5 =2
Graduation = 2ml
For cylinder C-
10-5 = 5
Number of spaces= 5
∴5/5 = 1
Graduation = 1ml
Hence , Graduations for A , B and C will be 0.2 ml, 2ml and 1ml respectively.
To learn more about graduated cylinders refer-https://brainly.com/question/26216613
#SPJ9
Your question is incomplete. Please find the missing image below.
What is the number of molecules in 1.00 mol of any gas?
Answers
The number of molecules in 1.00 mol of iodine gas is 6.02 × 10²³.
A mole is defined as the amount of a substance which contains 6.022 ×10²³ entities like atoms, ions, particles, molecules, etc. of the given substance. Mole will measures the number of ions, atoms, or molecules. The number of moles of a substance in a given pure sample will be represented by the following formula:
n = N/NA
Now, a mole is termed as very large collection of particles. In order to have 1.00
moles of iodine crystals, you need to have exactly 6.022 × 10²³ molecules of iodine.
1 mole of I₂ = 6.022 × 10²³ molecules of I₂ (Avogadro's constant)
We have, 1.00 moles of I₂ = 6.02 × 10²³ molecules of I₂.
To know more about mole here
https://brainly.com/question/29724957
#SPJ4
2 The name of CICH -CH-CH2OH is A. 1 Chloro propan-3-01 B. 3 chloro propan-1-01 C. 1-chloro propanol D. 3 chloro propanol
Answers
Answer:
3-chloro propan-1-ol
Explanation:
As long as you're writing IUPAC name of a compound, you must mark that the sequence of carbon atoms in the chain from alpha-carbon, which is the carbon right next to the -OH group in this case
What mass of chromium could be deposited by electrolysis of an aqueous solution of Cr2(SO4)3 for 155 min using a constant current of 10.0 A
Answers
The mass of chromium that could be deposited by electrolysis of an aqueous solution of Cr2(SO4)3 for 155 min using a constant current of 10.0 A is approximately X grams.
During the electrolysis process, the amount of substance deposited on an electrode can be determined using Faraday's laws of electrolysis. The first law states that the mass of substance deposited is directly proportional to the quantity of electricity passed through the electrolyte. The quantity of electricity can be calculated using the equation Q = I × t, where Q is the quantity of electricity in coulombs, I is the current in amperes, and t is the time in seconds. Since the current is given as 10.0 A and the time is 155 min (or 9300 s), we can calculate Q as Q = 10.0 A × 9300 s.
The next step is to determine the number of moles of chromium ions (Cr³⁺) in Cr2(SO4)3. Cr2(SO4)3 contains 2 moles of chromium ions per formula unit. To find the number of moles of chromium ions, we divide the quantity of electricity (Q) by the Faraday constant (F), which is approximately 96,485 coulombs per mole of electrons. Thus, the number of moles of chromium ions is Q / F.
Finally, to calculate the mass of chromium, we multiply the number of moles of chromium ions by the molar mass of chromium, which is approximately 52.0 g/mol. Therefore, the mass of chromium deposited is equal to the number of moles of chromium ions multiplied by the molar mass of chromium.
To learn more about electrolysis, click here:
brainly.com/question/33301115
#SPJ11
bigger size elements have low ionization energy and high shielding effect .justify the statement
Answers
Bigger size elements have low ionization energy and high shielding effect because:
Shielding effect:This effect describes the decrease in attraction between an electron and the nucleus in any atom with more than one electron shell. The more electron shells there are, the greater the shielding effect experienced by the outermost electrons.When shielding effect decreases the attraction force exerted by the nucleus increases on valence electrons.As a result, atoms will be larger.Find more information about Shielding effect here:
brainly.com/question/26366780
What shape is a prism?
Answers
Explanation:
A prism is a solid geometric figure with two identical parallel bases that are both polygons, and the lateral faces are parallelograms with pairs of opposite sides of equal length.
The mass of gas particles does not significantly affect the pressure of the container.
True
False
Answers
Answer: True
Explanation: Pressure is not dependent on mass.
The specific heat of nickel is 0.445 J/g degree Celsius. How much heat is required to heat a 168 gram piece of nickel from 15.2 degrees Celsius to 43.6 degrees Celsius?
Answers
ANSWER - 2123.184 J
Consider an experimental run at 273 K where the initial number of moles (n1) is actually 1.00 mol, and the final number of moles (n2) is 2.00 mol. Use the simulation to find the volume (V1) of 1.00 mol of helium at 273 K, and calculate the final volume (V2).
Express the volume to three significant figures, and include the appropriate units.
Answers
Answer: The volume \((V_{1})\) of 1.00 mol of helium at 273 K is 22.4 L and the final volume \((V_{2})\) is 44.8 L.
Explanation:
Given: \(T_{1}\) = 273 K, \(n_{1}\) = 1.00 mol
\(T_{2}\) = 273 K, \(n_{2}\) = 2.00 mol
At the standard pressure, 1 atm the value of \(V_{1}\) and \(V_{2}\) is calculated as follows.
\(V_{1} = \frac{n_{1}RT_{1}}{P}\\= \frac{1.00 mol \times 0.0821 Latm/mol K \times 273 K}{1 atm}\\= 22.4 L\)
Similarly,
\(V_{1} = \frac{n_{2}RT_{2}}{P}\\= \frac{2.00 mol \times 0.0821 Latm/mol K \times 273 K}{1 atm}\\= 44.8 L\)
Thus, we can conclude that the volume \((V_{1})\) of 1.00 mol of helium at 273 K is 22.4 L and the final volume \((V_{2})\) is 44.8 L.
Which statement about the carbon cycle is most true?
A. Energy is destroyed when organic matter decays.
B. The total mass of carbon on Earth is always increasing.
C. Carbon is lost from the cycle when it enters the atmosphere.
D. Carbon changes form, but the total amount on Earth remains the same
Answers
Answer:
its a.
Explanation:
The force generated by charged objects is called:
Answers
Answer:
coulomb force/ electrostatic force
Explanation:
attraction or repulsion of particles
Answer:
coulombe law or coulomb's universe square law