A student mixes 40.mL40.mL of 0.10MHBr(aq)0.10MHBr(aq) with 60.mL60.mL of 0.10MKOH(aq)0.10MKOH(aq) at 25°C25°C. What is the [OH−OH−] of the resulting solution? [OH−]=0.060M[OH−]=0.060M [OH−]=0.033M[OH−]=0.033M [OH−]=0.020M[OH−]=0.020M [OH−]=0.00000010M
Answers
The [OH⁻] of the resulting solution is 0.020M.
To find the [OH⁻], we can use the fact that at 25°C, Kw (the ion product constant of water) = [H⁺][OH⁻] = 1.0 x 10⁻¹⁴. First, we need to find the initial concentration of [H⁺] in the solution.
Since HBr is a strong acid, it will dissociate completely in water to form H⁺ and Br⁻. Therefore, the initial [H⁺] concentration will be equal to the initial concentration of the HBr solution, which is 0.10 M.
Next, we need to find the concentration of [OH⁻] that results from the reaction between H⁺ and OH⁻. Using the balanced chemical equation:
H⁺ + OH⁻ -> H₂OWe can see that the stoichiometry of the reaction is 1:1 between H+ and OH⁻. Since we know the initial concentration of [H⁺] and we have added an equal volume of 0.10 M KOH, which will provide an equal amount of OH⁻, we can use the formula:
[H⁺][OH⁻] = 1.0 x 10⁻¹⁴0.10 x 10⁻³ M x [OH⁻] = 1.0 x 10⁻¹⁴[OH⁻] = 1.0 x 10⁻¹⁴ / 0.10 x 10⁻³ M[OH⁻] = 0.020 MTo learn more about solutions, here
https://brainly.com/question/30665317
#SPJ4
Related Questions
Design a synthesis of 2-chloro-4-nitroanisole from benzene or any mono-substituted benzene.
Answers
To design a synthesis of 2-chloro-4-nitroanisole from benzene or any mono-substituted benzene, several steps are required. The synthesis can be achieved through a multi-step process involving different chemical reactions.
Firstly, the mono-substituted benzene needs to be converted into an anisole, which can be done by reacting it with methanol in the presence of a Lewis acid catalyst like aluminum chloride. This reaction is called the Williamson ether synthesis.
Next, the anisole can be chlorinated using thionyl chloride or phosphorus pentachloride to form 2-chloroanisole. This is a nucleophilic substitution reaction where the hydroxyl group in the anisole is replaced by a chlorine atom.
Finally, the 2-chloroanisole can be nitrated using a mixture of concentrated nitric acid and sulfuric acid to form 2-chloro-4-nitroanisole. This is a typical electrophilic aromatic substitution reaction where the nitro group is introduced onto the aromatic ring of the molecule.
Overall, the synthesis of 2-chloro-4-nitroanisole from benzene or any mono-substituted benzene involves the design of several chemical reactions, including the Williamson ether synthesis, nucleophilic substitution, and electrophilic aromatic substitution. These reactions require careful consideration of reaction conditions, reagents, and catalysts to achieve high yields and purity of the final product.
To know more about the 2-chloro-4-nitroanisole refer here :
https://brainly.com/question/10698236#
#SPJ11
18. An electric motor turns a belt that powers a pump. If this system is compared to the chemical reactions of the cell, which part represents
ATP?
the electric motor
the pump
the belt
Answers
Answer:
A. the electric motor
Explanation:
A cell is a biological molecule which is the basic and functional unit of life. Cells undergo series of processes to function appropriately. ATP is an acronym for adenosine triphosphate, which is the source of energy for various cell processes.
In the given mechanical system, the electric motor provides the energy required energy to drive the system. Therefore, the electric motor has the same major function of providing energy for the system as the ATP in a cell.
Use the particle model of matter to explain the difference in comprssiblity between liquids and gases
Answers
Answer:
Use the particle model of matter to explain why you can compress a gas easily, but you cannot compress a liquid very easily. The particles in a gas have very large spaces between them, so the particles can be 'squashed' closer together, meaning the gas can easily be compressed to take up a smaller volume.
Explanation:
Fill In the
476 nm = 4.76 x 10^? Cm
Recall that 1 nm 1 x 10 m and 1 cm = 1 x 10-2 m. You will want to memorize the metric conversion factors and those covered in this lesson!

Answers
Answer:
I gotthe answer
Explanation:
The explanation is This
Now writing the value in sentific term
It would look like
\(4.76 \times {10}^{2} \times {10}^{ - 9} \)
\(4.76 \times {10}^{2 - 9} \)
4.76
\(4.76 \times {10}^{ - 7} \)
Now in centimeter
\(4.76 \times {10}^{ - 5} \)
PLEASE MARK ME BRAINLIEST IF MY ANSWER IS CORRECT PLEASE

In the laboratory, concentrated hydrogen chloric acid reacted with aluminum. Hydrogen gas was collected over water at 25 degrees Celsius and had a volume of 355 cm33 at a total pressure of 750 mm Hg. The vapor pressure of water at 25 degrees Celsius is 24 mm Hg. Find the partial pressure of hydrogen gas.
Answers
Answer:
i dont no this one plz the question is hard
How can we prepare for climate change??
Plzzzzzzzz helppppppo
Answers
Answer:
WE start by having all the thing s that would be necessary at that time so we would have to worry about what we don't or won't have if you do before hand.
Explanation:
A The birthrate is higher than the death rate.
B The fertility rate is decreasing quickly.
C The immigration rate is higher than the emigration rate.
D The carrying capacity has been surpassed.

Answers
Answer:
A. The birthrate is higher than the death rate
Explanation:
I need help please!!!!!

Answers
Answer:
sorry..,...........
.....
I onlyI and II onlyII onlyII and III onlyI and III onlyIII only

Answers
The species that act as acid are the ones that donates H⁺ in the reaction.
Since we are working with an equilibrium, we have to consider both directions.
From left to right, we have H₂O turning into OH⁻ because it lost one H⁺, so H₂O is acting as an acid in the forward reaction.
From right to left, we have CH₃NH₃⁺ tunrning into CH₃NH₂ because it lost one H⁺, so CH₃NH₃⁺ is acting as an acid in the backwards reaction.
So, the species that act as acids are H₂O and CH₃NH₃⁺, I and II only.
What are some things that all plants need to grow?
Answers
Answer:
sunlight, water, carbon dioxide, soil, are the basics which you also have phosphorus and nitrogen
what do i do can u help:)
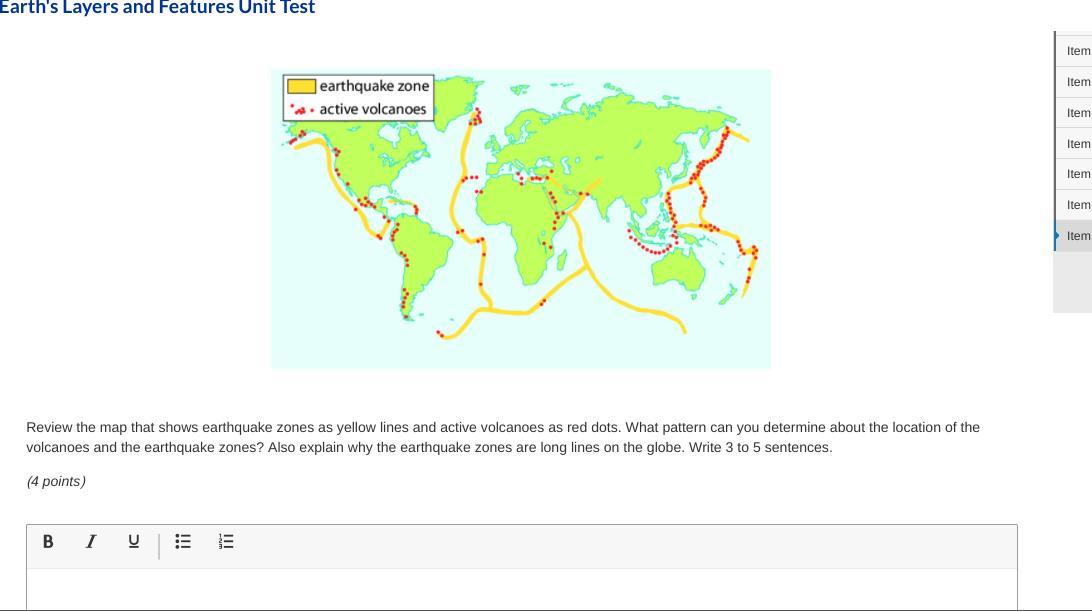
Answers
Answer:
The locations of the volcanoes tend to be close/on the earthquake lines/zones and this is because earthquake activity forms mountains/volcanoes and also causes those volcanoes to erupt. The reason the earthquake zones are long lines is because earthquakes usually happen on fault lines, or where two tectonic plates are next to each other. These plates are like a jigsaw puzzle, as in, they are everywhere making up the pieces of the surface of the globe. So that's why they are long lines.
In a double covalent bond, a carbon atom shares.
Answers
Answer:
Two pairs of electrons.
At STP, iodine, I2, is a crystal, and fluorine, F2, is a gas. Iodine is soluble in ethanol, forming a tincture of iodine. A typical tincture of iodine is 2% iodine by mass.
66 Compare the strength of the intermolecular forces in a sample of I2 at STP to the strength of the intermolecular forces in a sample of F2 at STP
Answers
At STP (Standard Temperature and Pressure), iodine is present in a crystalline form, whereas fluorine is in a gaseous form. Iodine is also soluble in ethanol and produces a tincture of iodine. Typically, a 2% iodine mass is present in a tincture of iodine.
The strength of the intermolecular forces in I2 (iodine) at STP is significantly higher than the strength of the intermolecular forces in F2 (fluorine) at STP. This is because of the difference in the bonding type, which is the primary factor that affects the strength of the intermolecular forces. Iodine is bonded covalently in its crystalline form, with every I2 molecule sharing electrons with another I2 molecule, making it a very strong intermolecular force. This bond is also known as a covalent bond. On the other hand, fluorine is bound by weak van der Waals forces due to its gaseous form, which are primarily dipole-dipole interactions. Since they are less polar, the van der Waals forces in F2 are weaker than in I2. These intermolecular forces are weaker because fluorine is in a gaseous form, while iodine is in a crystalline form. Hence, the strength of the intermolecular forces in I2 is much greater than the strength of the intermolecular forces in F2.For such more question on intermolecular
https://brainly.com/question/12243368
#SPJ8
At STP, iodine (I2) is a solid crystal and fluorine (F2) is a gas. A sample of I2 at STP is held together by van der Waals forces, which are weaker intermolecular forces. On the other hand, a sample of F2 at STP is held together by much stronger intermolecular forces than I2 due to its smaller size.
Therefore, F2 has stronger intermolecular forces than I2. It can be explained in a long answer as follows:At standard temperature and pressure, iodine (I2) is a solid crystalline substance. Its physical state is a solid because the intermolecular forces that bind the iodine molecules together are weak van der Waals forces. These forces are much weaker than chemical bonds, and they hold molecules in a condensed phase like a liquid or a solid. The forces of attraction between the iodine atoms in I2 are much weaker than the forces of attraction between the fluorine atoms in F2.
As a result, the boiling point of I2 is much lower than the boiling point of F2. F2 is a gas at STP since it is held together by much stronger intermolecular forces than I2 due to its smaller size. Fluorine has an electron density that is spread out over a larger area than iodine, making it more polarizable. The larger polarizability leads to stronger instantaneous dipoles and, as a result, stronger London dispersion forces. Since intermolecular forces are responsible for determining the physical state of a substance, F2 is a gas, whereas I2 is a solid. As a result, F2 has stronger intermolecular forces than I2.
To know more about intermolecular forces visit:-
https://brainly.com/question/31797315
#SPJ11
which compound, when stirred in water, will not pass through filter paper? (1) Hg2Cl2 (2) MgCrO4 (3) Na3PO4 (4) Na2S
Answers
The compound that will not pass through filter paper when stirred in water is (1) Hg₂Cl₂. This is because Hg₂Cl₂ is insoluble in water and forms precipitate when stirred in water. This precipitate will not pass through filter paper and can be separated from solution.
What is meant by compound?Compound is a substance that is made up of two or more different elements that are chemically bonded together in a fixed proportions. The elements in a compound cannot be separated by physical means, but can only be separated by chemical reactions. Compounds have their own unique chemical and physical properties that are different from the elements that make them up.
To know more about compound, refer
https://brainly.com/question/14782984
#SPJ1
Which of the following terms is a chemical substance made of a single type of atom that cannot be broken down into a simpler substance?
A- nucleus
B- molecule
C- Compound
D- Element
Answers
Explanation:
Elements are made of a single type of atom and cannot be broken down any smaller.
On treatment with dilute H2SO4, ethene forms ethanol.O TrueO False
Answers
The statement 'On treatment with dilute \(H_{2}SO_{4}\), ethene forms ethanol' is true as ethanol is the main product of this reaction.
The reaction between ethene and dilute H2SO4 produces ethanol. This reaction is an example of hydration reaction, in which water is added to a compound. In this case, the water molecule is provided by the dilute sulfuric acid (H2SO4). The reaction can be represented by the following equation:CH2=CH2 + H2O → CH3CH2OH.
In this reaction, the double bond between the two carbon atoms in ethene is broken, and a hydrogen atom and a hydroxyl group (-OH) are added to the two carbon atoms, forming ethanol. This reaction is typically carried out in the presence of a catalyst, such as phosphoric acid, to increase the rate of the reaction.
a piece of oak wood has a density of 0.921 g/cm3 and a mass of 7.31 g. what is the volume of this piece of wood? enter just the number - not the unit and round to 3 significant digits
Answers
The volume of this piece of oak wood is 7.93 cubic centimeters.
Density is defined as the mass per unit volume of a substance. The mass of the piece of oak wood is given as 7.31 g and the density is given as 0.921 g/cm³.
We can use the formula for density to find the volume of the piece of oak wood, which is given as: [Density = frac{Mass}{Volume}]
Rearranging the formula, we get:
[Volume = frac{Mass}{Density}]Substituting the given values, we get:
[Volume = frac{7.31 g}{0.921 g/cm³}]
Volume = 7.9271772058 cm³
Rounding to 3 significant digits, we get the volume of the piece of oak wood as:
Volume = 7.93 cm³
Learn more about volume from:
https://brainly.com/question/14197390
#SPJ11
7 types of chemistry
Answers
Answer:
Organic Chemistry.
Inorganic Chemistry.
Physical Chemistry.
Analytical Chemistry.
Stereochemistry.
Biochemistry.
Geochemistry.
Forensic Chemistry.
Explanation:
Question:
7 types of chemistry
Answer:
Inorganic Chemistry, Physical Chemistry, Analytical Chemistry, Stereochemistry, Biochemistry, Geochemistry, and Forensic Chemistry.
Explanation:
Inorganic chemistry is a branch of chemistry that deals with inorganic compounds. Physical chemistry is the branch of chemistry concerned with the application of the techniques and theories of physics to the study of chemical systems. Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. Stereochemistry is the branch of chemistry concerned with the three-dimensional arrangement of atoms and molecules and the effect of this on chemical reactions. Biochemistry is the branch of science concerned with the chemical and physicochemical processes and substances that occur within living organisms. Geochemistry is the study of the chemical composition of the earth and its rocks and minerals, and Forensic chemistry is the application of chemistry and its subfield, forensic toxicology.
I hope it helps you!
~XxBells is a cute girlxX~
#Learn with Brainly
The graph below shows how the temperature and volume of a gas vary when the number of moles and the pressure of the gas are held constant. How can the volume of the gas be increased if the pressure is constant?

Answers
Answer:
Option C. By increasing the temperature
Explanation:
From the graphical illustration above, we see clearly that the volume and temperature of the gas are directly proportional. This implies that as the temperature increases, the volume will also increase and as the temperature decreases, the volume will also decrease. This can further be explained by using the ideal gas equation as shown below:
PV = nRT
P is the pressure.
V is the volume.
n is the number of mole.
R is the gas constant.
T is the temperature.
PV = nRT
Divide both side by P
V = nRT/P
Since n and P are constant, the equation above becomes:
V & T
V = KT
K is the constant.
The above equation i.e V = KT implies that:
As T increases, V will also increase and as T decreases, V will also decrease.
Considering the question given above,
The volume of the gas can be increased if the temperature is increased.
Jen wants to make a fair comparison of the reactivity if the metals with hydrochloric acid. What are the 2 variables that must be kept constant?
Answers
Answer:
the concentration of hydrochloric acid
the temperature in heating
what is ionic compounds??
Answers
Answer:
The ionic compounds are chemical compounds composed of ions, which is held together by electrostatic forces termed ionic bonding.
Which property of matter is conserved in chemical reactions and shown by balanced equations?
Answers
The property of matter that is conserved in chemical reactions and shown by balanced equations is known as the Law of Conservation of Mass. According to this law, mass can neither be created nor destroyed in a chemical reaction; it can only be transformed from one form to another.For instance, when two substances are combined, they react and form a new substance.
The products that are formed contain the same number of atoms as the reactants, but in different configurations. To keep track of the number of atoms on either side of the equation, we use coefficients, which indicate the number of molecules or atoms of each substance in the reaction. However, when a chemical equation is written, it must adhere to the law of conservation of mass.The law of conservation of mass is critical in chemical reactions because it ensures that the amount of reactants that go into a reaction equals the amount of products that come out of it. This means that the total mass of reactants must equal the total mass of the products. As a result, the balanced chemical equation must reflect this law.For example, consider the reaction between hydrogen gas and oxygen gas, which forms water. The balanced chemical equation is as follows:2H2 + O2 → 2H2OIn this reaction, two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water. The coefficients in the balanced chemical equation indicate that two molecules of hydrogen and one molecule of oxygen combine to form two molecules of water, obeying the law of conservation of mass.In conclusion, the Law of Conservation of Mass is a fundamental principle in chemistry that is used to balance chemical equations. It is critical in chemical reactions because it ensures that the total mass of reactants equals the total mass of products, allowing scientists to accurately predict the outcome of a chemical reaction.For such more question on chemical reaction
https://brainly.com/question/11231920
#SPJ8
HELP ASAP
identify the three domains and the six kingdoms
Answers
Answer:
3 domains are Bacteria, Archaea, and Eukarya.
HELP.
Draw a graph of the average reaction time (variable y) versus the amount of water added (variable x) using your data from part B.


Answers
The graph is a graph of the average reaction time against the volume of water added.
How would the graph of the average reaction time and the volume of water added be plotted?The graph of the average reaction time and the volume of water is plotted with average reaction time on the Y-axis and the amount of water added on the x-axis.
The table is given below:
Average reaction time: 9.5 16.2 20.6 25.4
Volume of water added: 7.5 15 22.5 30
The graph is found in the attachment.
In conclusion, the graph provides a picture of the data provided.
Learn more about graphs at: https://brainly.com/question/19040584
#SPJ1

why might genetically identical twins have different phenotype
Answers
Answer:
They may have different phenotypes because of differences in their environments, such as nutrition and healthcare. Why might genetically identical twins have different phenotypes? Any genes on the same chromosome could be linked genes, whereas only genes on sex chromosomes can be sex-linked genes.
Explanation:
Due to the effects of the environment, genetically identical twins might have different phenotype.
What is phenotype?The qualities or visible characteristics of an organism are its phenotype. It depends on how the environment and the genotype, or genetic code, of the organism interact.
Although human monozygotic twins as well as other genetically identical species may share startling physical similarities, they frequently differ in key characteristics, such as complicated diseases. The effects of the environment have generally been blamed for this variance across organisms with essentially identical chromosomal DNA sequences.
Therefore, due to the effects of the environment, genetically identical twins might have different phenotype.
To know more about phenotype, here:
https://brainly.com/question/11197451
#SPJ2
____________ or _____________ serve as nutrients for plants.
please please help meee
Answers
Answer:
Carbon, hydrogen, nitrogen, oxygen, phosphorus,or potassium as nutrients for plants.
Explanation:
mark me as brainliest if it helped you
Carbohydrates or starch serve as nutrients for plants.
Metal objects, such as knifes or bullets, that come into contact with bones can leave trace evidence on them.
O True
O False
Answers
What does it mean if EROEI = 1? a. None of the above b. It's early days of fossil fuel exploration c. It's a perfect return on investment d. The efficiency is 100%
Answers
When EROEI (Energy Return on Energy Investment) is equal to 1, it means that the energy gained from a particular source is equivalent to the energy invested in obtaining that energy. In other words, the energy return is equal to the energy input. This indicates a situation where the energy extraction process is barely breaking even, with no net gain or loss in energy.
EROEI is a metric used to assess the efficiency and viability of energy sources. It measures the amount of usable energy obtained from a particular energy source divided by the amount of energy invested to extract or produce that energy. A value of 1 means that the energy gained is just enough to offset the energy invested.
In practical terms, an EROEI of 1 implies that the energy source being evaluated is not very efficient. It suggests that the amount of energy required to extract, process, or produce the energy is nearly equal to the energy obtained. Therefore, there is little to no surplus energy available for other uses or to sustain the energy extraction process itself.
An EROEI of 1 is often associated with energy sources in their early stages of development or exploration, where the technology or extraction methods may not be fully optimized. It could also indicate energy sources with high production costs or low energy density.
Learn more about EROE
brainly.com/question/32215499
#SPJ11
TNT is manufactured by the reaction of toluene with nitric acid according to the following equation: C7H8 (l) + 3 HNO3 (aq) ---> C7H5(NO2)3 (s) + 3 H2O(l)
Calculate the mass of TNT expected from the reaction of 69.0 g of toluene
Answers
Answer:
170. g C₇H₅(NO₂)₃
General Formulas and Concepts:
Chemistry - Stoichiometry
Reading a Periodic TableUsing Dimensional AnalysisExplanation:
Step 1: Define
RxN: C₇H₈ (l) + 3HNO₃ (aq) → C₇H₅(NO₂)₃ (s) + 3H₂O (l)
Given: 69.0 g C₇H₈ (Toluene)
Step 2: Identify Conversions
Molar Mass of C - 12.01 g/mol
Molar Mass of H - 1.01 g/mol
Molar Mass of N - 14.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of C₇H₈ - 7(12.01) + 8(1.01) = 92.15 g/mol
Molar Mass of C₇H₅(NO₂)₃ - 7(12.01) + 5(1.01) + 3(14.01) + 6(16.00) = 227.15 g/mol
Step 3: Stoichiometry
\(69.0 \ g \ C_7H_8(\frac{1 \ mol \ C_7H_8}{92.15 \ g \ C_7H_8} )(\frac{1 \ mol \ C_7H_5(NO_2)_3}{1 \ mol \ C_7H_8} )(\frac{227.15 \ g \ C_7H_5(NO_2)_3}{1 \ mol \ C_7H_5(NO_2)_3} )\) = 170.085 g C₇H₅(NO₂)₃
Step 4: Check
We are given 3 sig figs. Follow sig fig rules and round.
170.085 g C₇H₅(NO₂)₃ ≈ 170. g C₇H₅(NO₂)₃
What is the percentage composition for H2O in this compound Al(OH)3.2H2O?
a
50.55%
b
40.05%
c
32.73 %
d
65.09%
Answers
Answer:
32.73%
Explanation:
To solve this problem, first find the molar mass of Al(OH)₃.2H₂O
Atomic mass of Al = 27g/mol
O = 16g/mol
H = 1g/mol
Molar mass = 27 + 3(16 + 1) + 2(2(1) + 16)
= 27 + 51 + 36
= 114g/mol
Percentage composition = \(\frac{36}{114}\) x 100 = 32.73%