A student finds the mass and volume of four mystery liquids. The data is provided
Answers
The student's task is to determine the density of the four mystery liquids using the mass and volume measurements.
Density is a physical property that describes the amount of mass per unit volume.
The formula for density is density = mass/volume. Once the density of each liquid is determined, the student can compare it to known densities of different substances to identify the liquid.
This information can be useful in various fields such as chemistry, pharmacology, and environmental science.
The student may also use this data to calculate other properties of the liquids such as viscosity, surface tension, and boiling point. Overall, measuring mass and volume is a fundamental method in scientific research and analysis.
To know more about mystery liquids refer here: https://brainly.com/question/22053907#
#SPJ11
Related Questions
A compound contains only carbon, hydrogen, and oxygen. Combustion of 10.68 mg of the compound yields 16.01 mg and 4.37 mg . The molar mass of the compound is 176.1 g/mol. What are the empirical and molecular formulas of the compound
Answers
Answer: See below
Explanation:
n of CO2 = 0.364mmol
Mass of C = 0.364*12 = 4.368 mg
n of H2O = 184.37 = 0.243 mol
The compound has 2*0.243mmol of H
Mass of H = 0.486 mg
Mass of O = 10.68 − (4.368+0.486) = 5.826mg
Moles of O = 0.364
C:H:O Ratios
0.364 : 0.486 : 0.364
= 1 : 1.34 : 1
= 3 : 4 : 3
So the empirical formula is C3H4O3,
Empirical formula mass
= 88= 2 × Molar mass
And the molecular formula is C6H8O6
in which of these compounds are there twice as many oxygen atoms as hydrogen atoms?

Answers
Answer:
G - H2SO4
Explanation:
two hydrogen atoms and 4 oxygen atoms
One of the fastest supercomputers in the world is NEC's Earth Simulator. It can complete 40 trillion calculations per second! How long would it take this computer to perform 250 million calculations?
Answers
Answer:
0.0000625th of a second
Explanation:
divide (2.5 x 108) / (4.0 x 1013) = .625 x 10-5 = 0.0000625th of a second.
The molar heat of vaporization for liquid water is 40.6 kJ/mole.
How much energy is required to change 5.2 g of liquid water to steam if the water is already at 100oC?
Answers
So, if the water is already at 100oC, it would take 11,725 J of energy to convert 5.2 g of liquid water to steam.
For liquid water, the molar heat of vaporization is 40.6 kJ/mol4. If the water is already at 100°C, we can use the following formula to determine the amount of energy needed to convert 5.2 g of liquid water to steam:
Q = mL
What exactly is vaporization heat?
The quantity of enthalpy (heat energy) necessary to convert a liquid substance into a gas or vapour is known as the heat of vaporization1. Joules per mole (J/mole) or, in some cases, calories are used to measure it (C). Enthalpy of vaporization is another name for the heat of vaporization
m is the mass of water being vaporized (in grammes), Q is the needed energy, and L is the molar heat of vaporization (in J/mole).
https://brainly.com/question/29401184
To begin, we must change 5.2 g of water into moles:
18.015 g/mole / 5.2 g = 0.288 mole
Then, we can determine Q:
Q is equal to 0.28 mole, 40.6 kJ/mole, and 1000 J/kJ.
Q = 11,725 J
To know more about vaporization heat visit:
brainly.com/question/29401184
#SPJ1
When are these words used?
could ,would and should
what's difference between them?
Make 3 sentences using each word
Answers
a sentence for 'could': could u pass me the salt?
a sentence for 'would': what would you do if u were the president of usa?
sentence for 'should': you should practise ur spellings before the exam. :)
hope this helps!
Which of the following best describes the relationship between gas and volcanic
eruptions?
A The more gas magma contains, the more explosive a volcanic eruption will be.
B
The presence of gas in magma decreases the likelihood of a volcanic eruption.
As gas builds up in magma, volcanic eruptions occur once in the magma chamber
and once above ground
Gas found in magma determines only the intensity of eruptions involving
composite volcanoes and not shield volcanoes.
D
Answers
Answer:
A
Explanation:
The more gas magma contains, the more explosive a volcanic eruption will be.
What is the formula for beryllium cyanide?
BeCN2
Be(CN)2
BeCN
Be2CN
Answers
Answer:
B
Explanation:
I think tha one is more used because it's more reasonable
What is the molar solubility of lead iodide, PbI2, (in [Pb2+])Ksp = 9.8 × 10-9
Answers
The molar solubility of PbI2 of approximately 1.32 x \(10^-3 M.\) The molar solubility of lead iodide, PbI2, can be determined using the solubility product constant (Ksp) which is equal to 9.8 x \(10^-9\) for PbI2.
The Ksp is a measure of the equilibrium concentration of the ions in a saturated solution of the salt. In order to find the molar solubility, we need to use the Ksp expression which is given by Ksp = \([Pb2+][I-]^2.\)
Since the solubility of PbI2 will result in an equal concentration of Pb2+ and I-, the expression can be simplified to Ksp = \([Pb2+]^3.\) Rearranging the expression, we can solve for the molar solubility of PbI2 which is the concentration of Pb2+ ions in a saturated solution.
This means that at equilibrium, the concentration of Pb2+ ions in a saturated solution of PbI2 will be approximately 1.32 x \(10^-3 M.\)
To know more about molar solubility refer here:
https://brainly.com/question/28170449#
#SPJ11
for the reaction below: a. estimate the gas phase enthalpy change using bond dissociation enthalpies from the owl chemistry tables/appendix (button on the left). include algebraic sign and units. b. is the reaction exothermic or endothermic? c. is the reaction likely to proceed spontaneously in the direction written? : mark this question for later review.
Answers
a) DH^ r x n=-1∅7 k/ m o l
b) As DH is negative so it is exothermic.
c) it proceeds spontaneous in forward direction.
A) ch3 ch3
| |
CH3-C-CH3 +Br^2 .CH3-C-CH3
| |
H Br
+H-BR.
DH^ r x n=Σ Bonds^ broken-Bonds^ formed
=DH(C-H)+DH(Br-Br)
=-DH(C-Br)+DH(H-Br)
=263+192-(366+276)
DH^ r x n= 455-642
DH^ r x n=-1∅7 k/ m o l
b) As DH is negative so it is exothermic.
c) it proceeds spontaneous in forward direction.
The liquid/gas phase change, like the solid/liquid phase change, requires energy. The enthalpy of vaporization (or heat of vaporization) is the amount of energy required to convert a liquid to a gas,
denoted as H v a p.
To learn more about gas enthalpy
https://brainly.com/question/13161589
#SPJ4
If 50. 0gS is allowed to react as completely as possible with 105. 0g F2
Answers
There would be an excees of 20.48 g of sulfur left.
What is the stoichiometry?
We would have to apply stoichiometry so as to solve the problem
We have that;
Number of moles of S = 50 g/32 g/mol
= 1.56 moles
Number of moles of F2 = 105 g/ 38 g/mol = 2.76 moles
Given that;
1 mole of S reacts with 3 moles of F2
1.56 moles of S reacts with 1.56 * 3/1
= 4.68 moles
F2 is the limiting reactant
Amount of sulfur reacted = 1/3 * 2.79
= 0.92
Excess sulfur = 1.56 moles - 0.92 = 0.64 moles
Mass of excess sulfur = 0.64 * 32 g/mol
= 20.48 g
Learn more about stoichiometry:https://brainly.com/question/28780091
#SPJ4
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: S(g) + 3F2(g) ->SF6(g) If 50 g S is allowed to react as completely as possible with 105.0g F2(g), what mass of the excess reactant is left.
Two liters of hydrogen gas are stored at a
pressure of 100 kPa. If the temperature
does not change, what will the volume
of the gas be when the pressure is
decreased to 25 kPa?
Answers
Answer:
the volume will expand
Explanation:
gas under pressure contracts, and expands with a lesser pressure
The volume of the hydrogen gas at 25 kPa is 8 Liters.
Explanation:
Given:
At constant temperature, 2 liters of hydrogen gas at 100kPa pressure.
To find:
The volume of the hydrogen gas at 25kPa.
Solution:
The initial pressure of the hydrogen gas =\(P_1=100 kPa\)
The initial volume of the hydrogen gas at 100 kPa =\(V_1=2 L\)
The final pressure of the hydrogen gas =\(P_2=25 kPa\)
The final volume of the hydrogen gas at 25kPa = \(V_2=?\)
Using Boyles law:
\(P_1V_1=P_2V_2\\100kPa\times 2 L=25 kPa\times V_2\\V_2=\frac{100 kPa\times 2L}{25 kPa}=8L\)
The volume of the hydrogen gas at 25 kPa is 8 Liters.
Learn more about Boyles law here:
brainly.com/question/12835309?referrer=searchResults
brainly.com/question/1437490?referrer=searchResults
find the name for this W(C2H3O2)4
Answers
The name for this W(C2H3O2)4 is the chemical formula for lead(IV) acetate, often known as lead tetraacetate, is Pb(C2H3O2)4.
What is chemical formula?Chemical formula is defined as any of a number of ways to describe the structure or content of chemical substances. The chemical formula is a way of expressing information about the atomic proportions that make up a certain chemical compound or molecule using the numbers and symbols of the chemical elements. The sorts of atoms and their numbers in a molecule or compound are described using chemical formulae. Each element's atoms are denoted by one or two distinct letters.
It is not a salt because it is a colorless solid that dissolves in nonpolar, organic liquids. Moisture causes it to break down, therefore acetic acid is usually added when storing it. The substance is utilized to create organic compounds.
Thus, the name for this W(C2H3O2)4 is the chemical formula for lead(IV) acetate, often known as lead tetraacetate, is Pb(C2H3O2)4.
To learn more about chemical formula, refer to the link below:
https://brainly.com/question/11995171
#SPJ2
Arena is investigating a separation technique using the following apparatus. salt solution and/or sugar solution. a) 2 beakers b) tripod stand and wire gauze c) glass rod i) name the separation technique she may be possibly investigating. ii)do you think she has missed out a very important apparatus? if yes, identify and state the importance of the apparatus.
Answers
Arena might be investigating a separation technique known as evaporation, but she is missing a burner to perform it properly.
i) Evaporation is a separation technique that can be used to separate a substance dissolved in a liquid. It is usually applicable when the solvent doesn't have a too high boiling point and the solute is not prone to degradation at increased temperature.
ii) However, as the method requires heating the solution in order to accelerate the evaporation of the solvent, and while the tripod stand and wire gauze are used to mount the beaker with the solvent, they are useless without a burner that is used to introduce heat.
You can learn more about evaporation here:
brainly.com/question/5019199
#SPJ4
Arrange this isoelectronic series in order of increasing radius: Brâ, Se2â, Sr2+, and Rb+.
Answers
The isoelectronic series refers to a group of ions or atoms that have the same number of electrons. In this case, the isoelectronic series consists of Br²⁺, Se²⁺, Sr²⁺, and Rb⁺.
To arrange these ions in order of increasing radius, we need to consider the effective nuclear charge and the number of electron shells. The effective nuclear charge is the positive charge felt by an electron due to the attraction of the nucleus and the shielding effect of other electrons. The number of electron shells determines the distance between the nucleus and the outermost electrons.
As we move from left to right across the isoelectronic series, the effective nuclear charge increases due to the addition of protons in the nucleus. This results in a stronger attraction between the nucleus and the electrons, leading to a decrease in the atomic radius. As we move from top to bottom in the series, the number of electron shells increases, resulting in an increase in the atomic radius.
Based on this information, we can arrange the isoelectronic series in order of increasing radius as follows:
Sr²⁺ < Rb⁺ < Se²⁺ < Br²⁺
The smallest ion in the series is Sr²⁺ because it has the highest effective nuclear charge and the fewest number of electron shells. The largest ion in the series because it has the lowest effective nuclear charge and the most electron shells.
Learn more about isoelectronic series here
https://brainly.com/question/31744361
#SPJ11
if you mistakenly extract the solution first with naoh (aq), and then with nahco3(aq), what results you will observe and why?
Answers
The NaOH extraction step would remove some acidic components, while the NaHCO3 extraction step may have limited effect if the significant acidic components have already been neutralized.
If you mistakenly extract a solution first with NaOH (aq) and then with NaHCO3 (aq), you would observe the following results:
NaOH Extraction:
When NaOH (aq) is added to the solution, it will react with acidic components present in the solution, such as carboxylic acids, phenols, or acidic functional groups. This reaction results in the formation of water-soluble salts or compounds, which will dissolve in the aqueous NaOH solution. As a result, the acidic components will be removed from the solution.
NaHCO3 Extraction:
When NaHCO3 (aq) is added to the remaining solution from the previous step, it will react with acidic components that were not neutralized by NaOH. NaHCO3 is a weaker base compared to NaOH and is primarily used to extract acidic compounds such as phenols and carboxylic acids. These acidic components will react with NaHCO3 to form water-soluble salts, which will dissolve in the aqueous NaHCO3 solution.
However, if NaOH is mistakenly used first, it is possible that some acidic components in the solution may have already reacted and been removed in the previous step. Therefore, the NaHCO3 extraction step may not yield significant additional changes or observable results.The results of mistakenly extracting the solution first with NaOH (aq) and then with NaHCO3 (aq) would depend on the nature and concentration of the acidic components present in the solution.
Learn more about carboxylic acids visit:
brainly.com/question/4721247
#SPJ11
I kinda need help with this
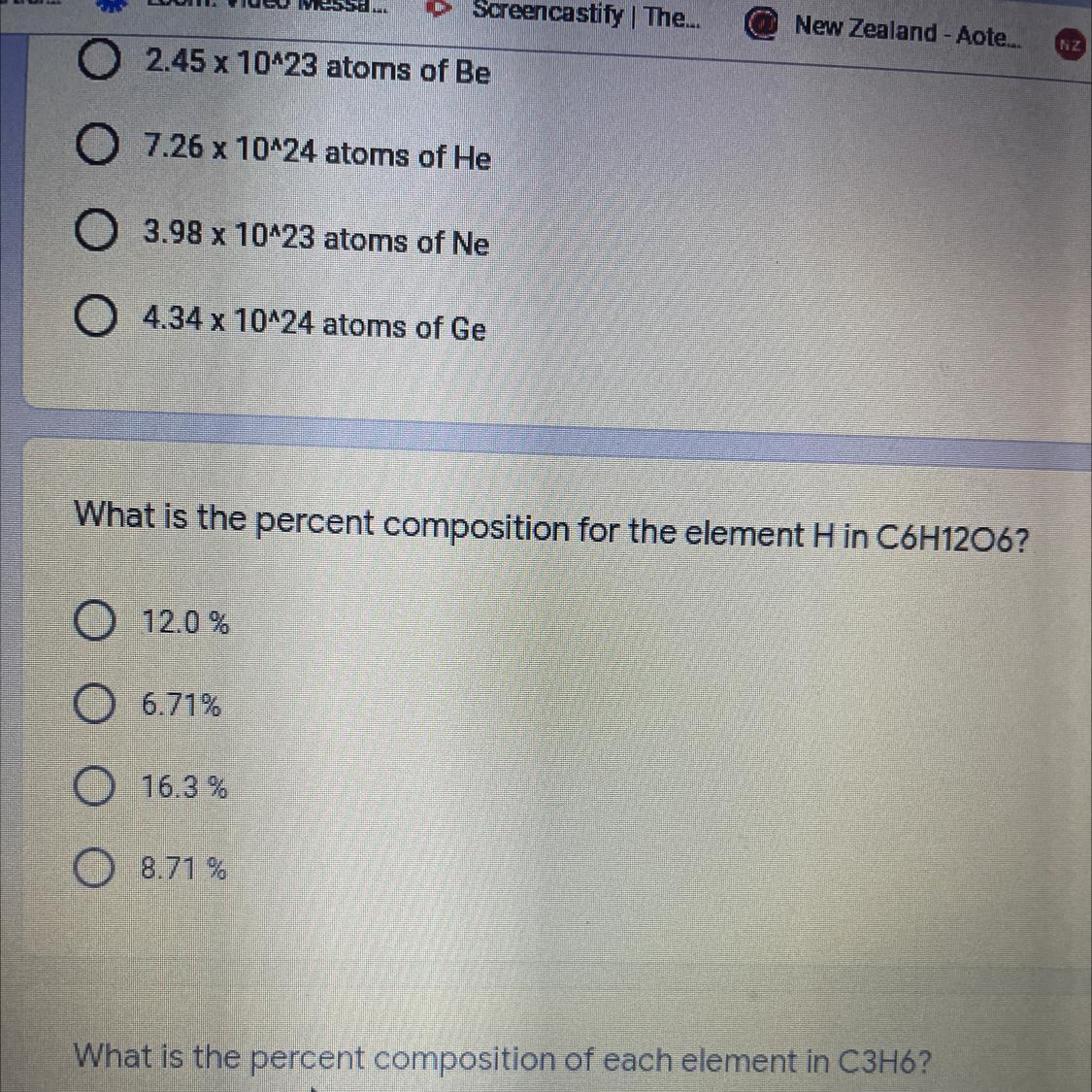
Answers
in oxidative phosphorylation what flows through the cytochrome chain
Answers
In oxidative phosphorylation, electrons flow through the cytochrome chain. The process begins with the transfer of high-energy electrons from reduced molecules, such as NADH and FADH2, to protein complexes in the inner mitochondrial membrane called the electron transport chain (ETC).
Electrons move along the ETC through various protein complexes, including cytochromes, which are heme-containing proteins that facilitate electron transfer.
As electrons flow through the cytochrome chain, they lose energy, which is used to pump protons across the inner mitochondrial membrane, creating an electrochemical gradient.
This proton gradient drives ATP synthesis via ATP synthase, an enzyme that utilizes the potential energy stored in the gradient to generate ATP from ADP and inorganic phosphate. This coupling of electron transport to ATP synthesis is called oxidative phosphorylation.
To know more about oxidative phosphorylation refer here:
https://brainly.com/question/29104695#
#SPJ11
Who was the leader of Iraq? *
Osama Bin Laden
Mikael Gorbachev
Şaddam Hussein
Answers
Answer: I believe it was Şaddam Hussein
Explanation:
energy transfer by electromagnetic waves
Answers
Answer:
Radiation is the transfer of heat energy through space by electromagnetic radiation.
Explanation:
Radiation is the transfer of heat energy through space by electromagnetic radiation. Most of the electromagnetic radiation that comes to the earth from the sun is in the form of visible light. Light is made of waves of different frequencies.
Factors identified as associated with (and possibly causing) type 1 diabetes mellitus include all of the following EXCEPT;
a) autoimmune reaction
b) absolute deficiency of insulin
c) dysfunctional insulin receptors
d) genetic factors
Answers
The factor identified as not associated with (and possibly causing) type 1 diabetes mellitus is option c) dysfunctional insulin receptors.
Insulin-producing cells in the pancreas are destroyed in type 1 diabetes mellitus, an autoimmune condition. Type 1 diabetes mellitus is thought to be caused by or be influenced by the following factors:
Autoimmune response: An inadequate supply of insulin results from the immune system wrongly attacking and destroying the pancreatic beta cells that produce insulin.Total lack of insulin: When beta cells are destroyed, the body experiences a total lack of insulin because the generation of insulin is either drastically decreased or stopped.Genetic factors: Type 1 diabetes has a strong hereditary component, and some genetic variants can raise the likelihood of acquiring the disease.A hormone called insulin is produced by beta cells in the pancreas. It is essential for controlling blood sugar levels and making it easier for cells to absorb glucose for use as fuel. Insulin signals cells in the liver, muscle, and fat tissues to absorb glucose from the bloodstream, assisting in the maintenance of normal blood sugar levels.
To learn more about insulin, refer to:
https://brainly.com/question/786474
#SPJ4
what is the full electron configuration for phosphorus, atomic symbol P 
Answers
Answer: 1s^2 2s^2 2p^6 3s^2 3p^3.
Explanation:
Breaking it down:
The first energy level (1s) contains 2 electrons.
The second energy level (2s) contains 2 electrons.
The second energy level (2p) contains 6 electrons.
The third energy level (3s) contains 2 electrons.
The third energy level (3p) contains 3 electrons.
In total, phosphorus has 15 electrons, with the last electron occupying the 3p orbital.
The full electron configuration for phosphorus is 1s2 2s2 2p6 3s2 3p3.
The full electron configuration for phosphorus is a description of how its electrons are arranged in its atomic orbitals. It is represented as a series of numbers and letters that indicate the energy level, sublevel, and number of electrons in each sublevel. The full electron configuration for phosphorus is 1s2 2s2 2p6 3s2 3p3. This means that the first energy level contains two electrons, the second energy level contains eight electrons, and the third energy level contains five electrons. The "s" and "p" represent the different sublevels within each energy level, and the superscripts indicate the number of electrons in each sublevel.
To learn more about electronic configuration,
https://brainly.com/question/29184975?referrer=searchResults
https://brainly.com/question/29157546?referrer=searchResults
What are the uses of Diamonds?
your own answer
please help
Answers
Answer:
Diamonds are very important in our daily lives ,so therefore it has many uses
1.They are used ad beauty products
2.They are used in automative industry
3.They are used in making windows
4.They are used as medicine
An aqueous solution of 4 mol/L nitric acid is electrolysis in an electrolytic cell using graphite electrodes.
Name the gas given off at the anode
Answers
The gas given off at the cathode is hydrogen while the gas given off at the anode is oxygen.
What is electrolysis?The term electrolysis has to do with the decomposition of a substance by the passage of a direct current through it.
The ions present in the system are\(H^+\), \(OH^-\) and \(NO_3^-\) ions. The gas given off at the cathode is hydrogen while the gas given off at the anode is oxygen.
The half-reaction equation of the reduction is;
\(2H^+(aq) + e\) → \(H_2(g)\)
The oxidation half-reaction equation is;
\(4OH^- (aq)\) → \(2H_2O(l) + 4e\)
The overall equation is;
\(4H^+(aq) + 4OH^- (aq)\) → \(4H_2(g) + 2H_2O(l) + 4e\)
Learn more about electrolysis:
brainly.com/question/12054569
#SPJ1
a flask contains four gases: ch4, o2, c2h5, and n2. when the stopper is removed, which gas will diffuse the fastest? responses c2h5 uppercase c subscript 2 end subscript uppercase h subscript 5 end subscript o2 uppercase o subscript 2 end subscript ch4 uppercase c h subscript 4 end subscript n2
Answers
The gas that will diffuse the fastest is O2 (oxygen). Gases diffuse from areas of high concentration to areas of low concentration. Oxygen has a relatively small molecular size compared to the other gases.
So, it is more likely to move quickly through the container and escape through the opening. Methane (CH4) and Ethane (C2H5) are larger, and thus will diffuse slower than oxygen.
Nitrogen (N2) is an even larger gas, and it will diffuse the slowest of all the gases in the flask.
Methane is a chemical composite with the chemical formula CH₄. It is a group-14 hydride, the simplest alkane, and the main basic of natural gas.
To know more about Gases:
https://brainly.com/question/29665974
#SPJ4
Can someone please help with this Thermochemical Equation
The balanced thermochemical equation for the combustion of methane gas is:
Calculate much heat is released when 4.5 moles of methane gas undergo a combustion reaction.

Answers
The heat that is released by 4.5 moles of methane gas is 4005 kJ.
What is combustion?The chemical reaction of combustion involves the breaking of chemical bonds in the fuel molecules, followed by the recombination of atoms with oxygen to form new molecules such as carbon dioxide, water vapor, and other combustion products.
We know that the balanced reaction equation have been shown in the image that is attached here.
As such we have that;
1 mole of methane gas produces 890 kJ of heat
4.5 moles of methane gas would produce 4.5 * 890/1
= 4005 kJ
Learn more about combustion:https://brainly.com/question/15117038
#SPJ1
if trial 3 gave a corrected volume of 21.1 ml h2 at stp, and the student calculated that 9.64x10-4 moles of h2 should be generated in the reaction, what is the molar gas volume for this trial?
Answers
21.5 ml is the molar volume of gas for this trial.
Volume=21.1 ml
moles=9.64x10^-4
no. of moles= given volume/22.4 L
V=n×22.4 L
V=9.64x10^-4×22.4×1000 ml
V=21.5 ml
The equation relates the volume and number of moles of a gas. V is for volume, n is for the number of moles, and V is for molar volume. A proportionality constant known as the molar volume describes the volume of one mole of any gas at a specific temperature and pressure.
It is helpful to establish a standard temperature and pressure that may be used as reference conditions when comparing various gases since the volume and, consequently, the density of a gas rely on those two factors. The definitions of standard temperature and pressure are 0°C and 1 atm, respectively. Standard temperature and pressure are referred to as STP together.
To know more about molar volume visit : https://brainly.com/question/4172228
#SPJ4
URGENT CAN SOMEONE ANSWER THIS QUESTION AND SHOW THEIR WORK PLEASE! How many moles of ammonia (NH) can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen?
Answers
Answer:
0.116 moles of ammonia can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen.
Explanation:
The balanced chemical equation for the reaction of hydrogen and nitrogen to form ammonia is:
N2(g) + 3H2(g) → 2NH3(g)
To determine how many moles of ammonia can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen, we need to use the ideal gas law equation:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
First, we need to convert the volume of hydrogen gas to moles using the ideal gas law equation:
n = PV/RT
where P is the pressure in atm, V is the volume in liters, R is the gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
n = (1.2 atm)(4.0 L)/(0.0821 L·atm/mol·K)(50.0°C + 273) = 0.174 mol H2
Since there is excess nitrogen, all of the hydrogen will react to form ammonia. Using the mole ratio between NH3 and H2 from the balanced chemical equation:
2 mol NH3 / 3 mol H2
we can calculate how many moles of NH3 will be produced:
n(NH3) = (0.174 mol H2) × (2 mol NH3 / 3 mol H2) = 0.116 mol NH3
Therefore, 0.116 moles of ammonia can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen.
When multiplying numbers in scientific notation what do you do with exponents?
Answers
Answer:
Subtract them.
Explanation:
''''"Since all number in scientific notation have base 10, we can always multiply them and divide them. To multiply two numbers in scientific notation, multiply their coefficients and add their exponents. To divide two numbers in scientific notation, divide their coefficients and subtract their exponents."""""
I was actually learned about this in school just found an source.
Chemistry problem, I’m conflicted between A or D

Answers
If the balloons are placed in a warmer room, all of the balloons will increase in volume equally because they have equal numbers of molecules.
The correct answer is D.
What happens to the volume of gases when they are heated?According to the ideal gas law, the volume of a gas is directly proportional to its temperature when the pressure and number of moles are held constant.
When the balloons are placed in a warmer room, the temperature increases resulting in an increase in volume. Since all three balloons have the same number of molecules and experience the same increase in temperature, they will all increase in volume equally.
Learn more about the volume of gases at: https://brainly.com/question/25736513
#SPJ1
Consider the chemical equation.
CuCl2 + 2NaNO3 Right arrow. Cu(NO3)2 + 2NaCl
What is the percent yield of NaCl if 31.0 g of CuCl2 reacts with excess NaNO3 to produce 21.2 g of NaCl?
Use Percent yield equals StartFraction actual yield over theoretical yield EndFraction times 100..
49.7%
58.4%
63.6%
78.7%
Answers
Percent yield = 78.7% , the correct answer is D) 78.7%, which represents the percent yield of NaCl in the reaction.
To calculate the percent yield of NaCl in the given chemical equation, we need to compare the actual yield of NaCl with the theoretical yield. The theoretical yield is the amount of NaCl that would be produced if the reaction went to completion based on stoichiometry.
First, we need to determine the theoretical yield of NaCl. By examining the balanced equation, we can see that the stoichiometric ratio between CuCl2 and NaCl is 1:2. This means that for every 1 mole of CuCl2, 2 moles of NaCl are produced.
Step 1: Convert the mass of CuCl2 to moles using its molar mass.
Molar mass of CuCl2 = 63.55 g/mol (atomic mass of Cu) + 2 × 35.45 g/mol (atomic mass of Cl)
Molar mass of CuCl2 = 134.45 g/mol
Moles of CuCl2 = 31.0 g / 134.45 g/mol ≈ 0.231 mol
Step 2: Use the stoichiometry to calculate the theoretical yield of NaCl.
Since the stoichiometric ratio between CuCl2 and NaCl is 1:2, the moles of NaCl produced will be twice the moles of CuCl2.
Moles of NaCl (theoretical) = 2 × 0.231 mol = 0.462 mol
Step 3: Convert the moles of NaCl to grams using its molar mass.
Molar mass of NaCl = 22.99 g/mol (atomic mass of Na) + 35.45 g/mol (atomic mass of Cl)
Molar mass of NaCl = 58.44 g/mol
Theoretical yield of NaCl = 0.462 mol × 58.44 g/mol ≈ 26.96 g
Now, we can calculate the percent yield using the formula:
Percent yield = (Actual yield / Theoretical yield) × 100
Percent yield = (21.2 g / 26.96 g) × 100 ≈ 78.7%
Option D
For more such questions on Percent yield visit:
https://brainly.com/question/14714924
#SPJ8