a solution containing a kcl dissolved in water will have a than that of pure water. multiple choice higher boiling point and a lower freezing point none of the answers can be determined with the information provided. lower boiling point and a lower freezing point lower boiling point and a higher freezing point higher boiling point and a higher freezing point
Answers
A solution containing a KCl dissolved in water will have a higher boiling point and a lower freezing point than that of pure water.
Elevation in boiling point is a phenomenon that describe the boiling point of a liquid will be higher when another compound is added. Which means a solution has higher boiling point than pure solvent .
Depression in freezing point is a phenomenon that describe the freezing point of a liquid will be lower when another compound is added. Which means a solution has lower freezing point than pure solvent.
Thus, The boiling point and the freezing point of a solution of KCl in water will be greater and lower, respectively, than that of pure water.
To learn more about Boiling & Freezing point, Here :
https://brainly.com/question/7197568?referrer=searchResults
#SPJ4
Related Questions
Natural abundances vary based on the planet and its formation history. For example, Mars and Earth have different oxygen isotope ratios. It has been suggested that the moon was formed when a planet-sized object struck a glancing blow on Earth. Although the cores remained largely intact, the remnants of these two bodies eventually reformed into Earth and moon.
Answers
Answer: Planetary scientists have long believed that our moon formed following a collision between Earth and another planet, but studies of Earth and moon rocks suggest ... in its history, Earth was struck a glancing blow by a Mars-sized planet. ... Oxygen, for example, comes in different varieties, called isotopes.The Giant Impact, as pictured in a painting by William K. Hartmann on the ...
Explanation: I hope this was very helpful and A Mars-sized object known as Theia (“mother of the Moon”) crossed into Earth's orbit, ... If this giant impact theory is correct, there should be evidence ... which suggest Theia struck Earth with a glancing blow, the new work implies ... and 18O/16O ratios, scientists can infer different planetary origins by taking ..
What is the molar ratio of acid to base for the neutralization reaction between hcl and naoh?.
Answers
The mole ratio of acid to base when neutralizing hydrochloric acid with sodium hydroxide is 1:1. A mole of NaOH would completely neutralize one mole of HCl.
The mole ratio would be 2:1 if the hydrochloric acid and barium hydroxide were to be combined instead. Assuming they react in a 1:1 ratio in accordance with the balanced neutralization equation, the moles of acid and base are identical at the equivalence point in a neutralization. Using the reaction between solutions of hydrochloric acid and sodium hydroxide as an example, let's examine how a neutralization reaction creates both water and a salt. This reaction's general equation is NaOH + HCl H2O and NaCl.
Learn more about neutralization here-
https://brainly.com/question/27891712
#SPJ4
True or False: The exact location of an electron can be measured thanks to
modern science.
Answers
Answer:
false you can not get a exact location of electrons from just modern science
Write the formula of the coordination compound pentaamminecarbonatocobalt(III) iodide. Enclose the coordination complex in square brackets, even if there are no counter ions. Do not enclose a ligand in parentheses if it appears only once. Enter water as H2O. Use any abbreviation given in blue below, and enclose it in parentheses if there is more than one.
Answers
Answer:
[Co(NH3)5CO3]I3
Explanation:
The naming of coordination compounds follows certain rules specified by IUPAC. Usually, the name of the complex makes it quite easy to deduce its structure.
"Pentaamine" means that there are five NH3 ligands as shown in the structure. The ligand carbonato is CO3^2-. It has no prefix attached to it in the IUPAC name of the complex hence there is only one carbonato ligand present(recall that the complex has a coordination number of six). I did not enclose it within parenthesis as required in the question.
Lastly the III that appeared after the metal name "cobalt" shows its oxidation state. The iodide counter ions must then be 3 in number in order to satisfy this primary valency of the metal hence the inclusion of I3 in the structure of the complex.
Select the correct image
Trees with light brown bark dominate an ecosystem. The ecosystem has populations of insects that thrive on the tree bark. Over time, some
Insect species developed certain physical characteristics that helped them thrive on the tree bark. These physical characteristics also helped the
Insects defend themselves from predators. Which insect species is probably the species with the new physical characteristics?

Answers
Answer:
I think the insect would be the one in the second photo reading from left to right
Explanation:
the other insects:
the first of the first photo is a change of what would be the external surface of the insect, it is not the insect itself.
In the third photo, the insect has short legs with little grip, which indicates that it does not climb large areas on top, and finally, the 4 image is of an insect that lives in desert areas and is poorly coordinated in areas of high heat. and sand.
These are the reasons why I chose photo number two reading from left to right
Answer:
I would say that your answer would be the second one.( the red one)
Explanation:
because the legs it has seem like it would grip onto trees fairly well.
how do i solve this question? can i please get any help? id really appreciate it!:)
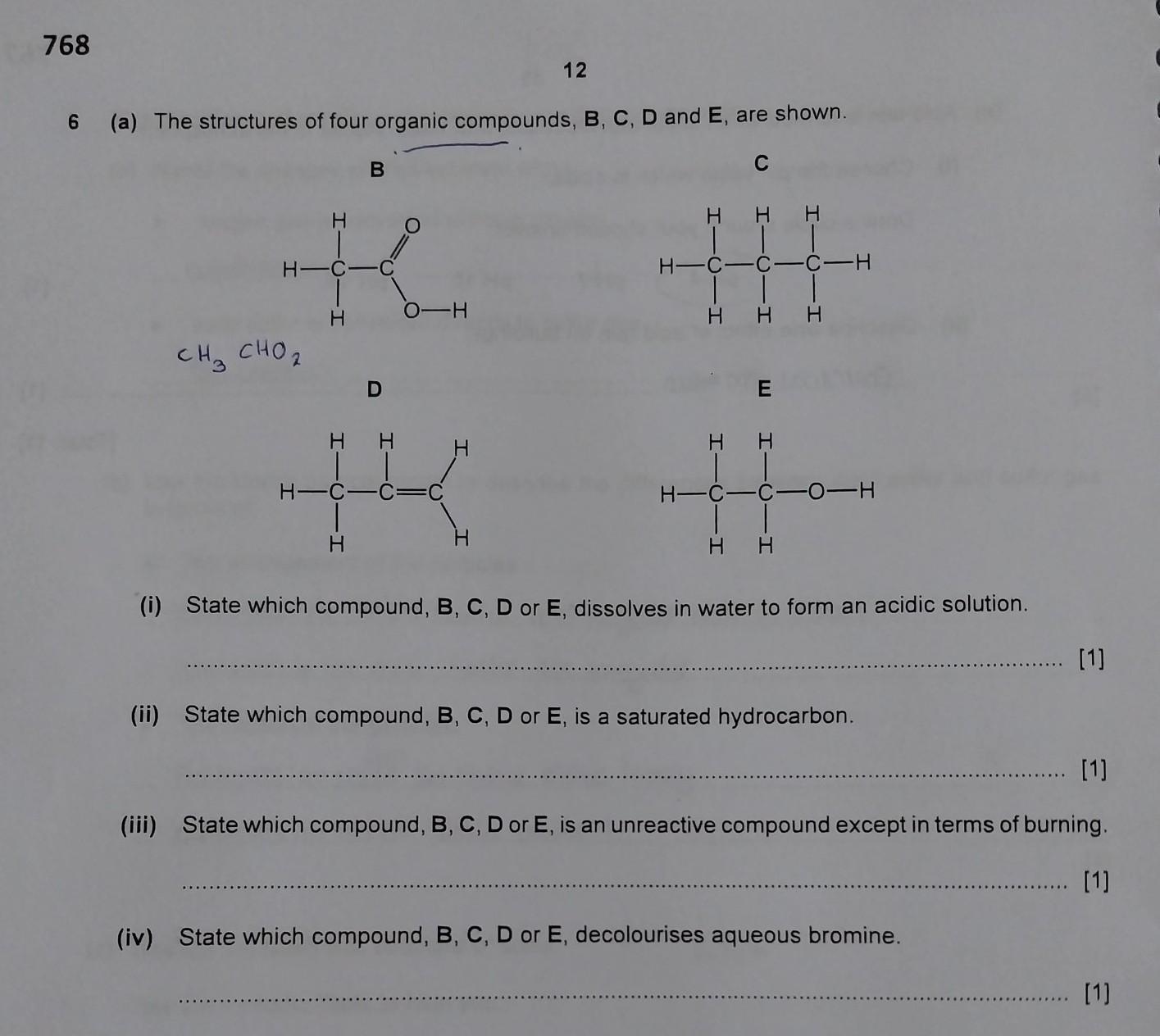
Answers
Answer:
(I) - B
(II) - C
(III) - C
(IV) - D
Explanation:
(I) both of carboxylic acids (B) and alcohols (E) are miscible in H2O, but carboxylic acids increase the acidity of water/solution
(II) Saturated hydrocarbons are from alkane hydrocarbons and they don't have pi bonds, only sigma bonds, and (C) is an alkane (Propane).
(III) Alkanes (C) are unreactive because of sigma bonds which are strong bonds that needs high energy to break
(IV) any unsaturated hydrocarbon (having pi bonds or double bonds, "==" in Lewis Structure) decolourizes Br2 since Br atoms react with the unsaturated hydrocarbon, producing bromo-alkane, for instance, 1-propene (D) + Br2 ---> 1-bromo propane
A 20.0 g sample is composed of 6.1 g of nitrogen (N) and 13.9 g of oxygen (O). What is the percent by mass of N in the sample?
A. 30.5%
B. 13.9%
C. 6.1%
D. 20.0%
Answers
A 20.0 g sample is composed of 6.1 g of nitrogen (N) and 13.9 g of oxygen (O). 6.1 % by mass of N in the sample. Therefore, option C is correct.
What does percent by mass mean?The mass percent of a solution is defined as the ratio of the mass of solute in a solution relative to the total mass of the solution.
Calculate the percent by mass of each element by dividing its mass in one mole of the compound by its molar mass and multiplying by 100%.
To calculate the percent by mass of N, we require dividing the mass of N in the sample by the total mass of the sample and multiply by 100.
% N = mass of N / total mass of the sample x 100
% N = 6.1 g / 20.0 g x 100
= 0.305 x 100
= 30.5%
Thus, option C is correct.
To learn more about the percent by mass, follow the link;
https://brainly.com/question/14990953
#SPJ1
The diagrams show objects’ gravitational pull toward each other. Which statement describes the relationship between
diagram X and Y?
A)Gravity attracts only larger objects toward one another
B)Gravity attracts larger objects only if they are dose to
one another
C)If the masses of the objects increase then the force
between them also increases,
D)If distance between the objects increases, then the
amount of force also increases
Need help plzzz

Answers
Answer:
Option C
Explanation:
The equation for showing gravitational pull or force between two objects is as follows -
\(G_f = \frac{G M_1M_2}{r^2}\)
Here Gf is the gravitational force
G is the gravitational constant and
M1 and M2 is the mass of the two objects
As we can see in the equation, that mass of two objects is directly proportional to the gravitational pull between them.
The higher the mass, the higher will be the force.
While on the other hand the distance between the two objects i.e "r" is inversely proportional. The higher the distance between the two objects the lower is the gravitational force between them.
Hence, option C is correct
What the answer please help

Answers
Answer:
cell,tissue ,organs,organism ,organ system
Answer:
cells, tissue, organ, organ system , and organism
Explanation:
Drag each positive ion to bond it with a negative ion to form the neutral ionic compound indicated.

Answers
Answer:
1. NaCl
2. NH4F
3. MgO
4.LiCl
5. KI
6. CaO
Explanation:
In that order
The addition of 3.15 g of Ba open parentheses OH close parentheses subscript 2 times 8 straight H subscript 2 straight O to a solution of 1.52 g of NH subscript 4 SCN in 100 g of water in a

Answers
The heat that is absorbed by the system is 1363 J. Option B
What is the heat absorbed?We know that in a chemical reaction that there could be the absorption or the evolution of heat. We say that there is the evolution of heat when heat has been lost from the system and there is the absorption of heat when heat has been gained by the system.
Number of moles of the barium hydroxide hydrate = 3.15 g/203 g/mol
= 0.015 moles
Number of moles of the ammonium thiocyanate = 1.52/76 g/mol
= 0.02 moles
If 1 mole of barium hydroxide hydrate reacts with 2 moles of ammonium thiocyanate
0.015 moles of barium hydroxide hydrate reacts with 0.015 * 2 moles/1 mole
= 0.03 moles
Hence the limiting reactant is the ammonium thiocyanate.
Now the heat that is absorbed is;
H = mcdT
m = mass of the water
c = Heat capacity
dT = Temperature change
H = 100 * 4.20 * 3.1
H = 1363 J
Learn more about heat capacity:https://brainly.com/question/28302909
#SPJ1
which of these substances are most likely crystalline solids?
Answers
Answer:
diamond
sugar
rubber
salt (NaCl)
wood
ice
flour
Answer:
diamond
sugar
salt
ice
Explanation:
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
how many significant figures do the following numbers have?1) 4.150 x 10-4
Answers
Significant figures correspond to the number of digits that a number contains. Zeros at the beginning and end of the number are not counted, only zeros are counted if they are in an intermediate position.
For this case, the number is written in scientific notation, the corresponding 10 of the scientific notation is not taken into account during the digit count, therefore the significant figures of this number will be:
Answer: So, in the number 4.150 x10^-4, there are 3 significant figures

What is the final temperature after 840 Joules is absorbed by 10.0g of water at 25.0
C?
Answers
The final temperature of the water is: T_final = 45.0°C
We can use the formula for the specific heat capacity of the water to solve this problem:
q = mcΔT
First, we can calculate the initial energy of the water:
q = mcΔT
q = (10.0 g) (4.184 J/g°C) (25.0°C)
q = 1,046 J
Next, we can calculate the final temperature after absorbing 840 J:
q = mcΔT
840 J = (10.0 g) (4.184 J/g°C) (ΔT)
ΔT = 20.0°C
Therefore, the final temperature of the water is:
T_final = T_initial + ΔT
T_final = 25.0°C + 20.0°C
T_final = 45.0°C
To know more about final temperature, here
brainly.com/question/11244611
#SPJ1
5) Read each Eco fact. Propose a solution to prevent the environmental problems of the
seaport of troy described in the eco fact.
Answers
Answer:
The creation of regulations that limit timber activities and the exploitation of wood can prevent soil erosion in Troy.
Explanation:
The exploitation of the wood was an intense activity and that did not have any regulation that would limit its damages, caused by its exploratory activities.
As the timber market was totally undisciplined, the exploitation of the wood caused a strong deforestation, leaving the soil totally unprotected and susceptible to strong erosion.
Soil erosion has a very negative impact on the environment, requiring regulations to be made to prevent this from happening.
Based on this, we can say that one way to prevent environmental problems in the seaport of troy is by establishing laws and regulations that limit logging activities.
In April, Earth's orbit makes the sun appear to be in the constellation Aries, even though the constellation itself is not visible during the day. In January, Earth's orbit makes the sun appear to be in the constellation Capricorn, even though the constellation itself is not visible during the day. The path the sun follows through the sky includes 12 constellations known as the zodiac.
What is the name given to this path or plane?
(1 point)
Responses
the ecliptic
the celestial equator
the zodiacal path
the solar orbit
Answers
Answer:
The ecliptic.
is the correct answer
Enzyme molecules are affected by changes in conditions within organisms. Explain how a prolonged, excessively high body temperature during an illness could be fatal to humans. Your answer must include:
the role of enzymes in a human
the effect of this high body temperature on enzyme activity
the reason this high body temperature can result in death
Answers
Enzymes are specialized proteins which can speed the reactions to a very great extent and thereby acts as efficient catalysts. The enzyme activity depends on pH and temperature.
What are enzymes?The biological catalysts which catalyze the chemical reactions taking place inside the cells and are capable of acting independently on the cells are defined as the enzymes.
Enzymes catalyze and control several chemical reactions that occur in our living cells. They helps in digestion, liver function, breathing, reproduction, nerve function, etc. The enzyme urea catalyzes the hydrolysis of urea.
The enzyme activity is sensitive to the temperature changes. All the enzymes shows maximum catalytic activity at optimum temperature. They are found to be denatured by heat and hence their activity is destroyed at high temperatures.
Thus at high temperature the enzymes will be inactive and this will affect the vital organs and lead to death.
To know more about enzymes, visit;
https://brainly.com/question/29774898
#SPJ1
If your equation includes 7(CrO4)2, how many Cr's are there?
If your equation includes 7(CrO4)2, how many O's are there?
Answers
If an equation includes 7(CrO₄)₂, the numbers of Cr's and O's atoms that are there are 14 and 56 respectively.
How to calculate number of atoms?The number of atoms present in a chemical compound can be calculated by multiplying the subscript of the particular element by any coefficient.
According to this question, 7 moles of chromate with the chemical formula; (CrO₄)₂ is given. The number of oxygen and chromium atoms in this compound can be calculated as follows:
Chromium = 7 (coefficient) × 2 = 14 atomsOxygen = 7 (coefficient) × 8 = 56 atomsLearn more about no of atoms at: https://brainly.com/question/14190064
#SPJ1
If you are given 96.0 grams of O2, how many grams of H20 are made?
Answers
Answer:
10.66 grams
Explanation:
why was the royal society of london formed
Answers
Answer:
..............what.......
Statements for a solution with a ph of 11.9
Answers
The acidity or alkalinity of a solution depends upon its hydronium ion concentration and hydroxide ion concentration. The pH scale is introduced by the scientist Sorensen. Here the given solution of pH 11.9 is basic.
The pH of a solution is defined as the negative logarithm to the base 10 of the value of the hydronium ion concentration in moles per litre. At 298 K, For pure water pH will be 7 and concentration of H₃O⁺ is 10⁻⁷M.
For a basic solution the pH will be greater than 7 and the concentration of H₃O⁺ will be less than 10⁻⁷M. For an acidic solution pH will be less than 7 and concentration of H₃O⁺ will be greater than 10⁻⁷M.
To know more about basic solution, visit;
https://brainly.com/question/15905763
#SPJ1
A solution with a pH of 11.9 is considered a basic or alkaline solution.
This indicates a high concentration of hydroxide ions (OH-) in the solution. The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 (most acidic) to 14 (most basic). A pH of 7 indicates a neutral solution, while a pH greater than 7 indicates a basic solution.
In practical terms, a solution with a pH of 11.9 could be found in various settings, such as in the alkaline batteries, household cleaning products, or industrial chemicals. Such a solution can be corrosive to metals and harmful to human skin and eyes. It is important to handle such solutions with care and appropriate protective equipment.
Learn more about pH, here:
https://brainly.com/question/491373
#SPJ1
It has been approximately 30 minutes since you
poured up the maxillary impression and base.
As you remove the impression from the model,
two anterior teeth break off. Discuss possible.
reasons why this happened
Answers
The breakage of the anterior teeth occurs because of there is not enough setting time, also a bubble or void in pour also.
What is dental impression models ?Imprints of teeth, gums, and other oral structures are captured in dental impressions. They are used to make dental restorations, whitening products, trays, retainers, mouth guards, and more. They are also used to build diagnostic models of your mouth. Digital or conventional dental imprints are both possible.
Patients who are prone to oral candidiasis might incorporate the dental imprint technique into their treatment plans to help manage infection. The denture base's proper lip support and fit enable for improved recording of the maxillomandibular jaw connection as well as opportunities to get around the limitations of the traditional method.
Here, the removal of the impression from the model leads to the breakage of anterior teeth its because, there is not enough setting time, also a bubble or void in pour also.
Find more on dental impression:
https://brainly.com/question/29563170
#SPJ9
What is the pH of a 0.0046 M nitric acid solution
Answers
Answer: 2.34
Explanation:
pH= -log (H+)
H+ is the acid's M
pH= -log (0.0046) =2.34
Given that 4 NH3 + 5 O2 → 4 NO + 6 H2O, if 3.00 mol NH3 were made to react with excess of oxygen gas, the amount of H2O formed would be
Answers
x mol H2O = 4.50 mol H2O
Step-by-step explanation:
From the balanced equation, we can see that for every 4 moles of NH3 that react, 6 moles of H2O are formed. Therefore, we can use a proportion to find the amount of H2O that would be formed if 3.00 mol of NH3 reacted:
4 mol NH3 : 6 mol H2O = 3.00 mol NH3 : x mol H2O
Solving for x, we get:
x mol H2O = (6 mol H2O / 4 mol NH3) * 3.00 mol NH3
x mol H2O = 4.50 mol H2O
Therefore, if 3.00 mol of NH3 were made to react with excess oxygen gas, 4.50 mol of H2O would be formed.
how does the process of photosynthesis convert carbon compound from p to carbon compound Q
Answers
The dark processes, a complicated series of enzymatically controlled chemical reactions, are what turn carbon into organic molecules. This word is rather misleading because these responses can occur in both light and darkness. As a result of extended darkness, some of the enzymes involved in the so-called dark processes become dormant; nevertheless, when the leaves that contain them are exposed to light, they become active.
The synthesis of intermediate sugar phosphates occurs in a cyclic process during the Calvin-Benson cycle, which involves the fixation, reduction, and use of carbon. Three molecules of carbon dioxide are used in a full cycle, which results in the production of one molecule of the three-carbon chemical glyceraldehyde-3-phosphate (Gal3P). Typically, the chloroplasts either export this three-carbon sugar phosphate or convert it to starch the chloroplast's inside.
The process of adding carbon dioxide to the five-carbon compound ribulose 1,5-bisphosphate (RuBP) and splitting the resulting six-carbon compound into two molecules of PGA is known as the initial incorporation of carbon dioxide, which is catalyzed by the enzyme ribulose 1,5-bisphosphate carboxylase (Rubisco). Each full turn of the cycle sees this reaction take place three times, resulting in the production of six PGA molecules.
The wavelength of light emitted from a traffic light having a frequency of 5.97×1014 Hz is ________ nm.
Answers
First, we have to remember the equation that relates the wavelength with the frequency:
\(v=\text{ }\lambda\times f\)In which v is the wave speed, lambda is the wavelength and f is the frequency.
From this equation, we can reformulate it as:
\(\lambda=\text{ }\frac{v}{f}\)Now, we know that the light speed is 3x108 m/s, so we can replace in the previous equation:
\(\lambda=\text{ }\frac{3\times10^8\text{ m/s}}{5.97\times10^{14}Hz}=\text{ 5.025}\times10^{-7}\text{ m = 502.51 nm}\)So, the answer is 502.51 nm
Define solubility. a solid that does not dissolve in a gas the amount of a substance that will dissolve in a given amount of solvent the amount of a substance that will dissolve in a given amount of solute a liquid that does not dissolve in another liquid a solid mixed with another solid
Answers
Answer:
the amount of a substance that will dissolve in a given amount of solvent.
Explanation:
Solubility is a term used to describe how readily a substance can be dissolved in a solvent to form a solution. Thus, a substance is said to be soluble if it dissolves completely in a solvent and insoluble if it doesn't dissolve or only dissolves partially.
For example, sodium chloride (NaCl) when mixed with water dissociates into sodium and chloride ions. Thus, salt (sodium chloride) is said to be soluble because it dissolves completely in water.
Furthermore, a compound that dissolves completely in water to produce an aqueous solution is said to be soluble in water.
In conclusion, solubility is simply the amount of a substance such as salt, that will dissolve in a given amount of solvent. A solvent is any liquid such as water, coffee, tea, etc., that dissolves a liquid, gaseous, or solid solute to produce a solution.
what is molarity of a stock solution that is 1.8 g/ml and 44% hcl
Answers
The molarity of the given stock solution is 0.02 M.
Molarity is the unit of concentration used for solution.
It is defined as the number of moles of solute per liter of solution.
The formula for molarity is given as: Molarity (M) = Number of moles of solute (n) / Volume of solution (V) in liters.
Here, we are given a stock solution having a density of 1.8 g/ml and 44% HCl. We are required to find the molarity of this solution.
To calculate the molarity, we need to first calculate the number of moles of solute present in the solution.
For that, we can use the formula:
Number of moles (n) = Mass (m) / Molar mass (Mm).
The molar mass of HCl is 36.46 g/mol (1 mole of HCl contains 1 mole of hydrogen and 1 mole of chlorine, which have atomic masses of 1.008 g/mol and 35.45 g/mol, respectively).
Let the mass of the solution be 'm'. Therefore, the mass of HCl present in the solution will be:
mass of HCl = 44% × mass of solution = 0.44m.
Since the density of the solution is 1.8 g/mL, we can find the volume of the solution as:
volume of solution = mass of solution / density
= m / 1.8 g/mL
= m / 1800 g/L.
Hence, the molarity of the solution can be calculated as:
Molarity = Number of moles of HCl / Volume of solution in liters
= (mass of HCl / Molar mass of HCl) / (volume of solution / 1000)
where volume of solution is divided by 1000 to convert mL to L.
Substituting the values, we get:
Molarity = (0.44m / 36.46 g/mol) / (m / 1800 g/L × 1000 mL/L)= (0.44 × 1800) / (36.46 × 1000)= 0.02 M.
for such more questions on molarity
https://brainly.com/question/30404105
#SPJ8
Which of the following makes sense to round to the nearest 10?
A. Your friend's telephone number
B. The PIN code of your city or town
C. The number of pages in your Maths book
D.t he year of your birth
Answers
Answer:
C
Explanation:
You can’t round a phone number or Pin code
why would you round your birth year?
Makes the most sense, you cannot round a phone number, your birth year, or a pin code