A solution consisting of 11. 4 g NH4Cl in 150 ml of water is titrated with 0. 20 M KOH.
a. How many milliliters of KOH are required to reach the equivalence point?
b. Calculate {Cl-], [K+], and [NH3] at the equivalence point. Assume volumes are additive
Answers
a.It requires 1066 mL of 0.20 M KOH to reach the equivalence point.
b.The equivalence point, the concentration of [Cl-], [K+], and [\(NH_{3}\)] in the solution, is 0.175 M.
What is the Equivalence point?
The chemical equivalent between the added titrant and the sample analyte is called the equivalence point in a titration.
a. We need to know how many moles of \(NH_{4}Cl\) are in the solution to calculate the volume of 0.20 M KOH needed to achieve the equivalence point.
First, we can determine how many moles \(NH_{4}Cl\) are present in the solution:
moles \(NH_{4}Cl\) = mass / molar mass
moles \(NH_{4}Cl\) = 11.4 g / 53.49 g/mol (molar mass of \(NH_{4}Cl\))
moles \(NH_{4}Cl\) = 0.2132 mol
At the equivalence point, all the \(NH_{4}Cl\) has interacted with the KOH, resulting in an equal amount of moles of \(NH_{3}\) and \(H_{2} O\). This suggests that 0.2132 moles of KOH are also needed to react with \(NH_{4}Cl\) The volume of 0.20 M KOH required to react with 0.2132 mol can be determined using the equation for the reaction between \(NH_{4}Cl\) and KOH:
\(NH_{4}Cl\) + KOH → \(NH_{3}\) + \(H_{2}O\) + KCl
moles KOH = moles \(NH_{4}Cl\)
= 0.2132 mol
volume of KOH = moles KOH / concentration of KOH
= 0.2132 mol / 0.20 mol/L
= 1.066 L or 1066 mL
Therefore, 1066 mL of 0.20 M KOH is required to reach the equivalence point.
b. At the equivalence point, an equal amount of moles of KOH and \(NH_{4}Cl\) interacted to create \(NH_{3}\), \(H_{2}O\), and KCl.
We may determine the concentration of [Cl-] and [K+] in the solution following the reaction at the equivalence point by assuming volumes are additive:
moles KCl = moles \(NH_{4}Cl\)
= 0.2132 mol
volume of solution = 150 mL + volume of KOH added
= 150 mL + 1066 mL
= 1216 mL
= 1.216 L
[Cl-] = moles KCl / volume of solution
[Cl-] = 0.2132 mol / 1.216 L
[Cl-] = 0.175 M
[K+] = moles KCl / volume of solution
[K+] = 0.2132 mol / 1.216 L
[K+] = 0.175 M
The fact that the reaction between \(NH_{4}Cl\)and KOH is a one-to-one reaction can be used to compute the concentration of [\(NH_{3}\)]. As a result, 0.2132 mol of NH3 is likewise created at the equivalence point. Using the overall volume of the solution, we can get the [\(NH_{3}\)] concentration:
[\(NH_{3}\)] = moles \(NH_{3}\)/ total volume of solution
[\(NH_{3}\)] = 0.2132 mol / 1.216 L
[\(NH_{3}\)] = 0.175 M
Therefore, at the equivalence point, the concentration of [Cl-], [K+], and [\(NH_3}\)] in the solution is 0.175 M.
Learn more about the Equivalence point from the given link.
https://brainly.com/question/2496608
#SPJ4
Related Questions
While you are conducting your experiment, you notice that the ball bounces at a different height and in a different direction for each trial. You look at the ground and see it is uneven. What could you do?
Answers
consider srh2 . does it require 2250 kj of energy to break one mole of the solid into its ions, or does breaking up one mole of solid into its ions release 2250 kj of energy?
Answers
The H oxidation number in these compounds is (-1).
An atom's oxidation state is indicated by the number assigned to it when it loses or gets an electron. It is consistently written in superscript.
An atom will reach a positive oxidation state when one of its electrons is lost. A negative oxidation state is what an atom will reach after it gains an electron.
Group-2 elements always have an oxidation state of +2.
Total hydrogen atom oxidation:
Each metal in the above compounds has an oxidation state of +2
Let x be the hydrogen atom's oxidation state.
Since the chemicals listed are neutral, their overall charge is zero.
To learn more about oxidation please click on below link
https://brainly.com/question/16976470
#SPJ4
In an ionic compound, the size of the ions affects the internuclear distance (the distance between the centers of adjacent ions), which affects lattice energy (a measure of the attractive force holding those ions together). Based on ion sizes, arrange these compounds by their expected lattice energy. Note that many sources define lattice energies as negative values. Please arrange by magnitude and ignore the sign. ∣∣lattice energy∣∣=absolute value of the lattice energy Na2S, K2S Cs2S Li2S
Answers
Answer:
Li2S> Na2S> K2S> CsS
Explanation:
The lattice energy of ionic species depends on the relative sizes of ions in the ionic compounds. As the size of ions increases, the lattice energy decreases and vice versa.
When the size of the anions are the same, the lattice energy now depends on the relative sizes of the cations. Therefore, since all the compounds are sulphides and the order of magnitude of ionic sizes is: Li^+ < Na^+ < K^+ < Cs^+.
Therefore, the order of decrease in lattice energy is; Li2S> Na2S> K2S> CsS
The electron configuration of an element is 1s22s2. How many valence electrons does the element have? (1 point) a 1 b 2 c 3 d 4
Answers
Answer:
b
Explanation:the shell is 2 so ignore the 1s electrons it is only the level 2 electrons that are valence
100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS


Answers
Answer:
2.2 moles
Explanation:
n = CV
n = 0.4×5.50
n=2.2 moles
Answer:
Explanation:
#2 is answered so here is for #3:
Knowns: 100mL of solution; concentration of 0.7M
Unknown: number of moles
Equation: number of moles = volume * concentration
Plug and Chug: number of moles = 100/1000 * 0.7 = 0.07 mole
Final Answer: 0.07mole
Under which conditions is a gas likely to experience the fewest intermolecular forces?.
Answers
Higher temperatures and lower pressures produce optimum gas behaviour. Because of the gas's intermolecular interactions, potential energy becomes significantly lower than kinetic energy under these circumstances.
What is potential energy?
Potential energy is a form of energy that is stored but is dependent on the relative positions of different system components. Stretching or compressing a spring increases its potential energy. A steel ball has more potential energy when it is raised above the surface of the earth than when it is brought to Earth.
Any object that is raised from rest has energy that can be released at a later time; for this reason, it is referred to as potential energy.
Therefore, Higher temperatures and lower pressures produce optimum gas behaviour.
To learn more about potential energy
Here: https://brainly.com/question/13997830
#SPJ4
A symbiotic relationship in which both organisms benefit is an example of
*
Mutualism
Commensalism
Competition
Answers
Mutualism is a type of symbiotic relationship where all species involved benefit from their interactions.
What is Symbiotic relationship?
The core of a symbiotic connection is the concept of aiding someone in exchange for benefit in return.
Symbiosis is the term used to describe tight relationships between two or more species. Because the two species involved in a symbiotic relationship coexist, it differs from typical interactions between species. Because this contact helps both species, symbiotic partnerships are prevalent among many creatures. However, certain symbioses are harmful to one or both of the species and are not helpful.
Mutualism happens when both species gain from a relationship. There are numerous mutualistic interactions because they are advantageous to both species involved.
Learn more about Symbiotic relationship from given link
https://brainly.com/question/20731481
#SPJ1
Create a model using images that would show what would happen to the igneous rock when it is exposed to different energy sources.
Answers
igneous is moved to Earth's surface and exposed to energy from the sun, it could weather into smaller rock pieces that could form sedimentary rock

D
C
1
2
3
4
5
6
7
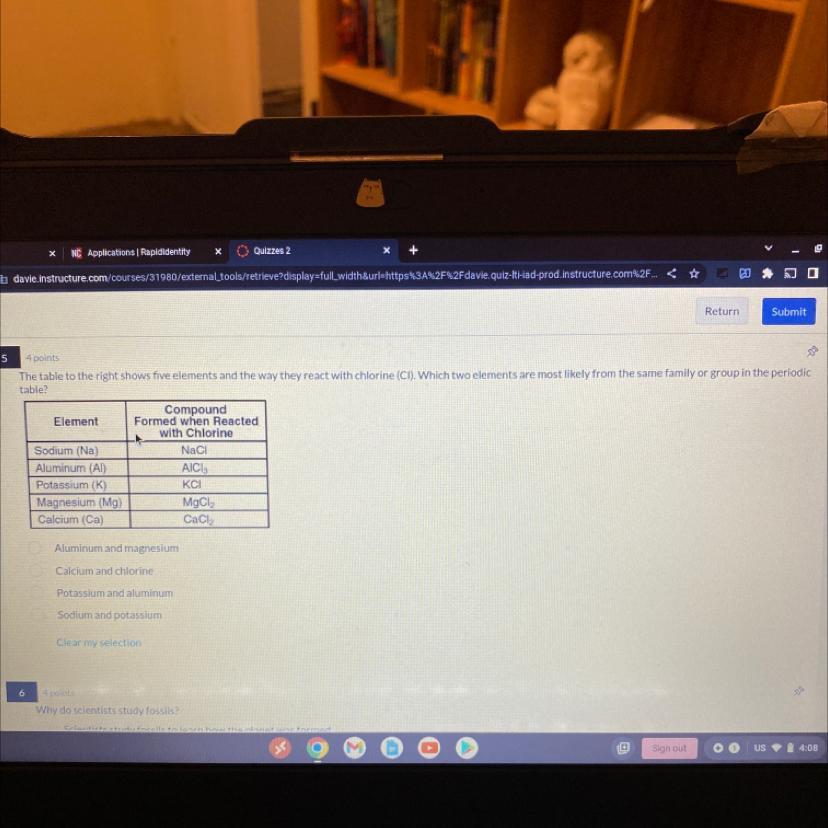
The table to the right shows five elements and the way they react with chlorine (CI). Which two elements are most likely from the same family or group in the periodic
table?
Element
Sodium (Na)
Aluminum (Al)
Potassium (K)
Magnesium (Mg)
Calcium (Ca)
Compound
Formed when Reacted
with Chlorine
Aluminum and magnesium
Calcium and chlorine
Potassium and aluminum
Sodium and potassium
Clear my selection
NaCl
AICI,
KCI
Mạch
CaCl₂
Answers
Sodium (Na) and Potassium (K) are most likely from the same family or group in the periodic table because they both react with chlorine (Cl) to form compounds that are similar in their chemical properties.
What is moles?Moles are a unit of measurement used in chemistry to express the amount of a substance. One mole of a substance is defined as the amount of that substance that contains as many elementary entities (such as atoms, molecules, or ions) as there are in 12 grams of carbon-12. Moles are used to simplify calculations involving chemical reactions and to relate the amount of one substance to the amount of another substance in a reaction. For example, the stoichiometry of a chemical reaction is expressed in terms of moles, where the coefficients in the balanced equation represent the number of moles of each substance that are involved in the reaction. Moles can be calculated from the mass of a substance using its molar mass, which is the mass of one mole of that substance.
Here,
Specifically, both Na and K react with Cl to form ionic compounds with a 1:1 stoichiometry, NaCl and KCl, respectively. This is because Na and K are both Group 1 (also called the alkali metals) in the periodic table, and they have similar electronic configurations, with a single valence electron in their outermost shell. This outer electron is easily removed, making them highly reactive with nonmetals like Cl. Aluminum (Al), Magnesium (Mg), and Calcium (Ca) are from different groups in the periodic table and do not form similar compounds with chlorine.
To know more about moles,
https://brainly.com/question/30763703
#SPJ1
Use the reaction to answer the question.
N₂(g) + 3H₂(g) + 2NH3(g) + energy
When this reaction has reached equilibrium, how will it respond when the temperature of the system increases?
(1 point)
O The concentration of N₂ will decrease.
O The concentration of H₂ will decrease.
O The equilibrium will shift to the left.
O The equilibrium will shift to the right.
Answers
The equilibrium will respond to an increased temperature of the system by shifting to the left. Option 3.
Le Charterlier's principle
According to Le Chatelier's principle, when a system at equilibrium is subjected to a stress, the system will respond in a way that tends to counteract the stress and reestablish equilibrium. In this case, an increase in temperature is a stress that will disrupt the equilibrium.
According to the reaction equation, the forward reaction is exothermic, meaning that heat is released when the reaction proceeds in the forward direction. Therefore, an increase in temperature will cause the equilibrium to shift in the direction that absorbs heat, which is the reverse reaction.
To counteract the increase in temperature, the equilibrium will shift to the left, favoring the reactants. This means that the concentration of N₂ and H₂ will increase, while the concentration of NH₃ will decrease. Therefore, the correct answer is: The equilibrium will shift to the left.
More on Le Chartelier's principle can be found here: https://brainly.com/question/29009512
#SPJ1
consider the case . you will see that there are degeneracies in the energy spectrum. some degeneracies have a simple symmetry explanation. if and are degenerate because of this symmetry, what is the relationship between them?
Answers
In the given case, where there are degeneracies in the energy spectrum, some of these degeneracies can be explained by simple symmetry. If states A and B are degenerate due to this symmetry, it implies that there is a relationship between them.
The relationship between the degenerate states A and B can be understood through the concept of symmetry operations. Symmetry operations are transformations that leave the physical properties of a system unchanged. These operations include rotations, translations, and reflections. If states A and B degenerate because of a specific symmetry operation, it means that this operation can transform state A into state B without changing the energy of the system. In other words, the symmetry operation connects the two degenerate states.
The specific relationship between states A and B depends on the nature of the symmetry operation involved. It could be a rotational symmetry, where the states are related by rotation. It could also be a translational symmetry, where the states are related by a translation. Alternatively, it could be a reflection symmetry, where the states are related by a reflection. In summary, if states A and B degenerate due to a symmetry operation, between them is established through that symmetry operation, which can be a rotation, translation, or reflection.
To know more about the energy spectrum please refer:
https://brainly.com/question/29061745
#SPJ11
A newly discovered gas, gas Q, travels 3.40 times faster than carbon dioxide. What is the molar mass of gas Q?
Answers
Answer:6.241
Explanation :Step 1: List the known quantities and plan the problem.
Step 2: Solve. n=PVRT=0.987atm×0.677L0.08206L⋅atm/K⋅mol×296K=0.0275mol. Now divide g by mol to get the molar mass. ...
Step 3: Think about your result. The R value that corresponds to a pressure in atm was chosen for this problem.
Select the most ideal gas situation:
Hydrogen and steam.
Answers
When hydrogen and steam are both present in a gas at the same pressure and temperature, this is the ideal gas condition. This is so because according to the ideal gas law, an ideal gas's pressure, volume, and temperature are all precisely proportional to one another.
This indicates that when the two gases have the same temperature and pressure, the two gases will also have the same volume. As a result, the gases are in their ideal state, having the same volume and pressure but retaining their distinct chemical compositions.
This is perfect because it enables the two gases to interact with one another in a predictable way, allowing for the measurement and prediction of the gases' behaviour.
Learn more about gases at:
https://brainly.com/question/1369730
#SPJ1
Which quantity is held constant when working with boyle’s, charles’s, and gay-lussac’s laws? volume moles pressure temperature
Answers
The quantity the is help constant in Boyles's law is Temperature, Charles' law is Pressure, Gay-Lussac's law is number of moles.
Boyle's law states that at constant temperature the volume of the gas is directly proportional to the pressure exerted by the gas.
P α V....(i)
Charles' law states that at constant pressure the volume of the gas is directly proportional to the absolute temperature of the gas.
V α T....(ii)
Gay-Lussac's law states that for a given number of molecules of gas the Pressure of the gas is directly proportional to the absolute Temperature of the gas.
P α T....(iii)
To know more about Boyle's law, here
https://brainly.com/question/6534668
#SPJ4
Realice una historieta que resuma su comprensión acerca de la teoría atómica y los diferentes modelos atomicos que se
han propuesto a lo largo de la historia.
Answers
Respuesta:
Los modelo atómicos han permitido representar el modo de funcionamiento de los átomos. A lo largo de la historia han surgido un numero de modelos atómicos diferentes incluyendo los modelos de Bohr, Thomson, Rutherford, Sommerfeld, Dalton y Schrödinger.
Explicación:
El modelo atómico propuesto por John Dalton (1808) demostró que las sustancias químicas reaccionan en proporciones fijas y cómo mediante su combinación se producen elementos diferentes. Dalton fue el primero en postular la existencia de elementos indivisibles llamados átomos. A continuación, Thomson (1904) desarrolló un modelo en el cual el átomo estaba compuesto por protones con carga positiva y electrones con carga negativa los cuales se incrustaban uniformemente dentro de este átomo, asemejándose a las pasas de uva de un budín. En 1911, Ernest Rutherford desarrolló un nuevo modelo donde la masa principal del átomo tenía carga positiva y se localizaban en el núcleo, mientras que los electrones con carga negativa se posicionaban en la región externa del átomo. Subsecuentemente, Niels Bohr (1913) represento el funcionamiento del átomo de hidrógeno mediante un protón inmóvil en el núcleo atómico y un electrón girando a su alrededor. El modelo atómico de Sommerfeld permitió generalizar el diagrama de Bohr a otros tipos de átomos mas allá del Hidrógeno, incluyendo diferentes niveles energéticos para cada átomo particular. El modelo de Schrödinger (1926) permitió corregir aquellas discordancias surgidas del modelo atómico de Bohr. Schrödinger incluyó diferentes niveles y subniveles de energía a los electrones e incorporó órbitas elípticas a su movimiento, con lo cual permitiendo predecir los efectos relativos de los campos magnético y eléctrico sobre el movimiento de los electrones.
A 4 feet tall student went summing pool. He saw depth of water in pool less than 4 feet.Will he drowned.Write reason
Answers
Answer:
Question says , the height of the student = 4 feet, ... This means, if the student goes for swimming in the pool, however he does not know swimming, he will not be drowned until he is suffering from an injury or external force.
They start with 0.0352 moles of cucl2(aq) and they conduct the various reactions described in the lab manual, which are given below. assuming no product is lost throughout the experiment, what is the theoretical yield of cu(s) in grams? the molar mass of cu is 63.546 g/mol. round your answer to 3 decimal places. do not include units in your answer.
Answers
Rounded to 3 decimal places, the theoretical yield of Cu(s) is 2.236 grams.
To calculate the theoretical yield of Cu(s) from 0.0352 moles of CuCl2(aq), we can use the stoichiometry of the reaction and the molar mass of Cu.
Since CuCl2 reacts to form Cu(s) in a 1:1 molar ratio, 0.0352 moles of CuCl2 will produce 0.0352 moles of Cu(s). Now, we can convert moles of Cu(s) to grams using the molar mass of Cu:
0.0352 moles Cu(s) * 63.546 g/mol = 2.236 grams of Cu(s)
Rounded to 3 decimal places, the theoretical yield of Cu(s) is 2.236 grams.
To learn more about mass, refer below:
https://brainly.com/question/19694949
#SPJ11
A gas mixture contains 1.52 atm of Ne, 766 mmHg of He and Ar. What is the partial pressure, in atmospheres, of At if the gas mixture has a total pressure of 3.27atm
Answers
Answer:
0.74 atm.
Explanation:
From the question given above, the following data were obtained:
Pressure of Ne (Pₙₑ) = 1.52 atm
Pressure of He (Pₕₑ) = 766 mmHg
Total pressure (Pₜ) = 3.27 atm
Pressure of Ar (Pₐᵣ) =?
Next, we shall convert the pressure of He from mmHg to atm. This can be obtained as follow:
760 mmHg = 1 atm
Therefore,
766 mmHg = 766 mmHg × 1 atm / 760 mmHg
766 mmHg = 1.01 atm
Finally, we shall determine the partial pressure of Ar. This can be obtained as follow:
Pressure of Ne (Pₙₑ) = 1.52 atm
Pressure of He (Pₕₑ) = 1.01 atm
Total pressure (Pₜ) = 3.27 atm
Pressure of Ar (Pₐᵣ) =?
Pₜ = Pₙₑ + Pₕₑ + Pₐᵣ
3.27 = 1.52 + 1.01 + Pₐᵣ
3.27 = 2.53 + Pₐᵣ
Collect like terms
3.27 – 2.53 = Pₐᵣ
Pₐᵣ = 0.74 atm
Thus the partial pressure of Ar is 0.74 atm.
how an eruption of a volcano
Answers
The bonding and molecular polarity in a molecule of Cl2 is best described as...
a. non polar/polar
b. polar/polar
c. non polar/ nonpolar
d. polar/ ionic
Answers
Answer:
The bonding and molecular polarity in a molecule of CI2 is best described as palar / polar
1. Calculate the amount of energy given off when 17 g of vapor at 102C condenses to 87C
Did a phase change occur? If so, which one? Is this endothermic or exothermic?
Answers
Answer:
- Amount of energy = -1066.92 J
- Yes, a phase change occurs from gaseous state to liquid state
- It is exothermic
Explanation:
Using Q = m × c × ∆T
Where Q = amount of energy
m = mass = 17g
c = specific heat capacity of water (4.184J/g°C)
∆T = change in temperature (87°C - 102°C = -15°C)
Hence, Q = m × c × ∆T
Q = 17 × 4.184 × (-15°C)
Q = -1066.92 J
- According to this question, vapor condenses i.e. gaseous form of water changes to liquid water, which involves a reduction in temperature. Hence, a change of phase occurs from gaseous state to liquid state.
- Since the change of phase occurs from a less orderly state (gas) to a more orderly state (liquid), there is a release of energy i.e. EXOTHERMIC. The amount of energy or Enthalpy change (∆H) is negative
Can someone talk me about molecular mass and moles?
Answers
For example NaCl,
the molar mass will be The mass of Na + the mass of Cl . The mass of NA is 22.98, and the mass of CL is 35.45 so when you add them the molar mass will equal 58.43.
That is molar mass .
Another example H20
In this case, if theres a subscript in the formula include it . This means the mass of H (hydrogen) is 1.00784, you will times that by 2 because the subscript is 2
So the MASS of H2 is 2.01568. Then you add it to the mass of oxygen which is 15.999 . Because the oxygen doesnt have a subscript you dont multiply the mass by anything . So the molar mass is 18.01.
Now moles , is just the coefficient basically ,
so in a chemical equation for example you have 2Na + Cl = 2NaCl (sodium chloride)
The moles of Sodium alone will be 2 due to the coefficient , and the moles of sodium chloride will also be 2 as they happen to have the same coefficient
Another example
2 Fe + 3 Cl2 = 2 FeCl3
The moles of Fe (iron) is 2 , the moles of Cl (chlorine) is 3 and the mole of FeCl3 is 2
Basically if they ask for the mole of an element or compound just look at the coefficient
Assume that 50. 0mL50. 0mL of 1. 0MNaCl(aq)1. 0MNaCl(aq) and 50. 0mL50. 0mL of 1. 0MAgNO3(aq)1. 0MAgNO3(aq) were combined. According to the balanced equation, if 50. 0mL50. 0mL of 2. 0MNaCl(aq)2. 0MNaCl(aq) and 50. 0mL50. 0mL of 1. 0MAgNO3(aq)1. 0MAgNO3(aq) were combined, the amount of precipitate formed would
Answers
The amount of precipitate formed in both cases will be the same. If 50.0 mL of 2.0 M NaCl(aq) and 50.0 mL of 1.0 M AgNO3(aq) are combined, the reaction will consume the same number of moles of each reactant as if 50.0 mL of 1.0 M NaCl(aq) and 50.0 mL of 1.0 M AgNO3(aq) were combined.
If 50.0 mL of 2.0 M NaCl(aq) and 50.0 mL of 1.0 M AgNO3(aq) were combined, the amount of precipitate formed would be the same as if 50.0 mL of 1.0 M NaCl(aq) and 50.0 mL of 1.0 M AgNO3(aq) were combined.
This is because the balanced chemical equation for the reaction between NaCl(aq) and AgNO3(aq) shows that the reactants are present in a 1:1 ratio. This means that for every 1 mole of NaCl(aq) that reacts, 1 mole of AgNO3(aq) is also consumed. The concentration of a solution is a measure of the amount of solute present in a given amount of solvent, and is typically expressed in moles per liter (M).
Since the concentration of a solution is proportional to the number of moles of solute present, a 2 M solution of NaCl(aq) contains twice as many moles of NaCl(aq) as a 1 M solution of NaCl(aq). Similarly, a 1 M solution of AgNO3(aq) contains the same number of moles of AgNO3(aq) as a 1 M solution of NaCl(aq).
Learn more about the amount of precipitate at
https://brainly.com/question/15192688?referrer=searchResults
#SPJ4
Why is eating animals to obtain energy considered exothermic
Answers
A 175 gram sample of a metal at 93.50c was added to 105 grams of water at 23.50c in a perfectly insulated container. the final temperature of the water and metal was 33.80c. calculate the specific heat of the metal in j/g0c.
Answers
The specific heat of the metal is 0.214 J/g°C.
When a metal and water are mixed in a perfectly insulated container, they reach a final temperature through heat transfer. In this case, the initial temperature of the metal is 93.50°C, while the initial temperature of the water is 23.50°C. The final temperature of the mixture is 33.80°C.
To calculate the specific heat of the metal, we can use the principle of conservation of energy. The heat lost by the metal (Qmetal) is equal to the heat gained by the water (Qwater). The formula for heat transfer is:
Q = m * c * ΔT
Where:
Q is the heat transferred
m is the mass of the substance
c is the specific heat
ΔT is the change in temperature
Let's denote the specific heat of the metal as cm and the specific heat of water as cw. The heat lost by the metal can be calculated as:
Qmetal = cm * mmetal * (Tfinal - Tinitial_metal)
The heat gained by the water can be calculated as:
Qwater = cw * mwater * (Tfinal - Tinitial_water)
Since the container is perfectly insulated, the heat lost by the metal is equal to the heat gained by the water:
Qmetal = Qwater
cm * mmetal * (Tfinal - Tinitial_metal) = cw * mwater * (Tfinal - Tinitial_water)
Rearranging the equation, we can solve for the specific heat of the metal:
cm = (cw * mwater * (Tfinal - Tinitial_water)) / (mmetal * (Tfinal - Tinitial_metal))
Substituting the given values:
cm = (4.18 J/g°C * 105 g * (33.80°C - 23.50°C)) / (175 g * (33.80°C - 93.50°C))
After evaluating the expression, the specific heat of the metal is calculated to be approximately 0.214 J/g°C.
Learn more about specific heat
brainly.com/question/31608647
#SPJ11.
In an atomic model that includes a nucleus, positive charge is
a
concentrated at multiple sites in an atom.
b
concentrated in the center of an atom.
c
spread evenly throughout an atom.
d
located in the space outside the nucleus.
Answers
What is the end result of the Krebs cycle?
Answers
According to question, oxaloacetic acid is the end result of the Krebs cycle .
The cycle transforms the chemical energy of acetyl coenzyme A (acetyl CoA) into nicotinamide adenine dinucleotide's reducing force (NADH). The tricarboxylic acid (TCA) cycle, also known as the Krebs or citric acid cycle, is the main source of energy for cells and a crucial stage in aerobic respiration.
The Krebs cycle, also known as the tricarboxylic acid cycle or the citric acid cycle, is the central component of cellular metabolism and is crucial for the production of both energy and biomolecules. It helps the synthesis of ATP while completing the sugar cleavage work that was started during glycolysis.
To know more about Krebs cycle visit :
https://brainly.com/question/13153590
#SPJ4
A reaction mixture in a 3.620-L flask at a certain temperature initially contains 0.7660g H2 and 96.80g I2 At equilibrium, the flask contains 90.20 g HI
Answers
Element with the largest electron cloud
Answers
Out of the answer choices, rubidium has the highest energy valence shell. With a single electron in the fifth energy level, krypton will have the highest number of energy levels of the group I elements listed.
kasama na po jan ang explenation
cold tcs food should be stored at an internal temperature of
Answers
Do not accept the food or throw the products away if the food temperatures fall outside of acceptable ranges or if TCS food exhibits signs of previous temperature abuse. Keep cold food at or below 41°F.
Do not accept the food or throw the products away if the food temperatures fall outside of acceptable ranges or if TCS food exhibits signs of previous temperature abuse. Keep cold food at or below 41°F. At supermarkets, eateries, and other establishments that sell ready-to-eat meals, customers are always demanding TCS food items. At the point of sale, people are pickier and look for foods from vendors they believe will provide a high-quality and safe product. Managers and employees need to be aware of all the supply chain locations where TCS foods are at risk for time and temperature excursions just for this reason.
Learn more about temperature here:
https://brainly.com/question/11464844
#SPJ4