A site in Pennsylvania receives a total annual deposition of 2.688 g/mof sulfate from fertilizer and acid rain. The ratio by mass of ammonium sulfate/ammonium bisulfate/sulfuric acid is 3.0/5.5/1.0. (c) If 10. km² is the area of an unpolluted lake 3 m deep and there is no loss of acid, what pH will the lake water attain by the end of the year? (Assume constant volume.)
Answers
According to the given statement pH would be attained in the year = 5.192
What is pH scale?A scale used to specify the acidity or basicity of an aqueous solution, originally denoting "potential of hydrogen." The pH of acidic solutions is lower than the pH of basic or alkaline solutions.
According to the given information:The formula of the dissociation of H2SO4
\(\begin{gathered}\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{HSO}^{-}+\mathrm{H}_3 \mathrm{O}^{+} \\\mathrm{HSO}_4^{-}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{SO}_4^{2-}+\mathrm{H}_3 \mathrm{O}^{+}\end{gathered}\\\)
As we can see ,
2 moles of \(\mathrm{H}_3 \mathrm{O}^{+}\) in total were produced from 1 mole \(\mathrm{H}_2 \mathrm{SO}_4\)
solve for the concentration of \(\mathrm{H}_3 \mathrm{O}^{+}\) in the lake.
Now solve for the number of moles.
Mole of \(\mathrm{H}_3 \mathrm{O}^{+}=9460 \mathrm{~kg} \mathrm{H} \mathrm{H}_2 \mathrm{SO}_4 \times \frac{1000 \mathrm{~g}}{1 \mathrm{~kg}} \times \frac{1 \mathrm{~mol} \mathrm{H}_2 \mathrm{SO}_4}{98.1 \mathrm{~g} \mathrm{H}_2 \mathrm{SO}_4} \times \frac{2 \mathrm{~mol} \mathrm{H}_3 \mathrm{O}^{+}}{1 \mathrm{~mol} \mathrm{H_{2 } \mathrm { SO } _ { 4 }}}\)
Mole of \(\mathrm{H}_3 \mathrm{O}^{+}=192864.4241 \text { moles } \mathrm{H}_3 \mathrm{O}^{+}\)
The solve for the volume of the lake:
V = lhw = Ah
= 10 km² x ((1000 m)²/(1 km)²)x3mx(1000 L/1 m³)
V = 3.0 x 10^10
Now solving for \(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]\)
\(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\frac{\mathrm{mol}}{L}=\frac{192864.4241 \mathrm{~mol} \mathrm{H}_3 \mathrm{O}^{+}}{3.0 \times 10^{10} \mathrm{~L}}\)
= 6.4288^-6 M
Solving for the pH
\(p H=-\log \left[H_3 O^{+}\right]\)
pH = -log(6.4288^-6)
pH = 5.192
pH would be attained in the year = 5.192
To know more about pH scale visit:
https://brainly.com/question/10825137
#SPJ4
Related Questions
Read the following statements and answer: i. Ozone protects us from sun’s harmful radiations. ii. Ozone absorbs the UV radiations from the sun and breaks down to oxygen iii. Oxygen acts as a protective shield. a. Statement i and ii are correct, but iii is false. b. Statement i and ii are false, but iii is correct. c. Statement i is true, ii and iii are false. d. All are correct statements.
Answers
Answer:
d. all statements are correct
Explanation:
..........................................................
Answers
Explanation:
...........................................................
Answer:
??????????????
Explanation:
where is your question?
Which of the following is an example of a behavioral trait that
is likely to increase reproductive success?
O colorful feathers
O a dance
O tail length
Answers
Answer:
The answer is option A
Colorful feathers
Hope this helps you
Calculate the concentrations of hydronium ion and hydroxide ion at 25°C in: (a) 0.10 M HCl, (b) 1.4 × 10–4 M Mg(OH)2, a strong base. answer with steps please
Answers
Ai. The concentration of hydronium ion, [H₃O⁺], is 0.10 M
Aii. The concentration hydroxide ion, [OH⁻] is 1×10⁻¹³ M
Bi. The concentration of hydronium, ion [H₃O⁺], is 3.57×10⁻¹¹ M
Bii. The concentration hydroxide ion, [OH⁻] is 2.8×10¯⁴ M
A. How do i determine [H₃O⁺] and [OH⁻] of 0.10 M HCl?i. The concentration of hydronium ion, [H₃O⁺] can be obtained as follow:
HCl(aq) + H₂O <=> H₃O⁺(aq) + Cl⁻(aq)
From the above equation,
1 mole of HCl contains 1 mole of H₃O⁺
Therefore,
0.10 M HCl will also contain 0.10 M H₃O⁺
Thus, the concentration of hydronium ion, [H₃O⁺] is 0.10 M
ii. The concentration of hydroxide ion, [OH⁻] can be obtained as follow:
Concentration of hydronium, ion [H₃O⁺] = 0.10 MConcentration hydroxide ion, [OH⁻] =?[H₃O⁺] × [OH⁻] = 10¯¹⁴
0.10 × [OH⁻] = 10¯¹⁴
Divide both side by 3.02×10⁻¹⁰
[OH⁻] = 10¯¹⁴ / 0.10
[OH⁻] = 1×10⁻¹³ M
Thus, concentration of hydroxide ion, [OH⁻] is 1×10⁻¹³ M
B. How do i determine [H₃O⁺] and [OH⁻] for 1.4×10¯⁴ M Mg(OH)₂?First, we shall obtain concentration hydroxide ion, [OH⁻]. Details below:
Mg(OH)₂(aq) <=> Mg²⁺(aq) + 2OH⁻(aq)
From the above equation,
1 mole of Mg(OH)₂ is contains 2 mole of OH⁻
Therefore,
1.4×10¯⁴ M Mg(OH)₂ will contain = 1.4×10¯⁴ × 2 = 2.8×10¯⁴ M OH⁻
Thus, concentration hydroxide ion, [OH⁻] is 2.8×10¯⁴ M
Now, we shall obtain the concentration of hydronium, ion [H₃O⁺]. Details below:
Concentration of hydroxide ion, [OH⁻] = 2.8×10¯⁴MConcentration of hydronium, ion [H₃O⁺] = ?[H₃O⁺] × [OH⁻] = 10¯¹⁴
[H₃O⁺] × 2.8×10¯⁴ = 10¯¹⁴
Divide both side by 2.8×10¯⁴
[H₃O⁺] = 10¯¹⁴ / 2.8×10¯⁴
[H₃O⁺] = 3.57×10⁻¹¹ M
Thus, the concentration of hydronium, ion [H₃O⁺], is 3.57×10⁻¹¹ M
Learn more about hydroxide ion concentration, [OH⁻]:
https://brainly.com/question/19800885
#SPJ1
How many bonds are broken in the combustion of one methane molecule?.
Answers
According to the number of bonds present in a methane molecule, in the combustion of one methane molecule, four C-H covalent bonds are broken.
How many bonds are broken in a methane molecule?A methane molecule is composed of four atoms of hydrogen and one atom carbon.
The formula of a methane molecule is CH4.
The bonds present in methane molecule are four C-H covalent bonds.
Therefore, in the combustion of one methane molecule, four C-H covalent bonds are broken.
Learn more about about methane at: https://brainly.com/question/25419929
What type of compound forms when a metal reacts with water?
Answers
Answer:
Metals react with water to form oxides or hydroxides and release Hydrogen gas.
Explanation:
Which of the following is a FALSE statement? *
A. Heat moves through solids by by conduction.
B. Molecules move faster in warmer substances.
C. Warm water is denser than cold water.
D. Heat moves through liquids and gases by convection.
Answers
Answer:
C: Warm water is denser than cold water
What is the mass of a 5.00 cm^3 piece of copper having a density of 8.96 g/cm^3
Answers
Answer:
44.8 g
Explanation:
Density = mass / Volume
Mass = density x Volume = 8.96x 5 = 44.8 g
HELP PLEASE
What term describes ocean water being forced onto land during a hurricane?
current
tsunami
storm surge
white cap
Answers
Answer:
Storm Surge
Explanation:
A storm surge is a rise in sea level that occurs during tropical cyclones, intense storms also known as typhoons or hurricanes.
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound
Answers
C₁H₂O₁ is the empirical formula for the substance. The substance has the molecular formula 4 (C₁H₂O₁).
How does empirical formula work?The empirical formula is the most basic whole number ratio of atoms in a given compound. The empirical formula is typically applied to simply display the elements that make up a molecule. This is helpful if you want to quickly identify the elements you're working with. The molecular formula comes in handy when you want to figure out how many atoms of each element are contained in the compound.
Element % Atomic mass Relative no. of atoms Simplest whole ratio
C 40.6 12 40.6/12 = 3.3 3.3/3.3 = 1
H 5.1 1 5.1/1 = 5.1 5.1/3.3 = 2
O 54.2 16 54.2/16 = 3.3 3.3/3.3 = 1
The compound's empirical formula is C₁H₂O₁ or CH₂O
Molecular formula = Empirical formula × n
n = Molecular weight/ Empirical weight
n = 118.034/30
n = 4
Molecular formula = n × Empirical formula
Molecular formula = 4 (C₁H₂O₁)
To know more about empirical formula visit:
https://brainly.com/question/28080770
#SPJ4
Which elements are most vigorous and which elements are least vigorous. (a) sodium and iodine. (b) lithium and iodine. (c) potassium and fluorine. (d) potassium and bromine. (e) sodium and bromine
Answers
The elements that are most vigorous would be (c) potassium and fluorine.
The elements that are the least vigorous would be (d) potassium and bromine
Which elements are reactive ?Potassium is a metal, less reactive than sodium but more reactive than lithium, and fluorine is a highly reactive nonmetal, so both elements would be considered vigorous.
Sodium is a highly reactive metal and bromine is a nonmetal, less reactive than iodine but more reactive than chlorine, so both elements would be considered vigorous but not as much as other options.
Find out more on vigorous elements at https://brainly.com/question/24155109
#SPJ1
which two gases are primarily responsible for the greenhouse effect because of their ability to absorb infrared energy?
Answers
The two primary gases responsible for the
greenhouse effect
are carbon dioxide (CO2) and water vapor (H2O). They absorb infrared energy, which is a type of energy that is emitted from the Earth's surface, and trap it in the atmosphere.
This energy can't escape, which causes the atmosphere to warm up, resulting in the greenhouse effect.
The greenhouse effect is a natural process that helps to keep the Earth's temperature relatively stable, which is important for life.
The amount of CO2 and H2O in the atmosphere are regulated by natural processes, such as respiration and
photosynthesis
,
but human activities, such as burning fossil fuels and deforestation, have caused these levels to increase significantly over the past few decades.
This has resulted in a further increase in the temperature of the atmosphere, leading to climate change.
CO2 absorbs more infrared energy than other gases, but H2O also plays an important role in the greenhouse effect.
H2O exists in the atmosphere in both vapor and liquid forms, and is able to absorb and trap heat energy more effectively than CO2.
H2O also has the ability to reflect incoming sunlight, which further helps to keep the temperature of the atmosphere warm.
CO2 and H2O are the two primary gases responsible for the greenhouse effect because of their ability to absorb infrared energy and trap heat in the atmosphere.
These two gases are essential for regulating the temperature of the Earth and maintaining the climate.
Human activities have caused their levels to increase, resulting in a further increase in the temperature of the atmosphere and leading to climate change.
to know more about
greenhouse effect
refer here:
https://brainly.com/question/13706708#
#SPJ11
In a 3.21g sample of the hydrate, CuSO4 • 10H2O (339.8 g/mol), how many grams of water are expected?
Answers
Therefore, 9.49 grams of water is expected in the given 3.21 g sample of CuSO4 • 10H2O.
To determine the number of water molecules in the given hydrate, CuSO4 • 10H2O, we'll need to find out the molar mass of the compound and the molar mass of water to make a comparison.
The molar mass of CuSO4 • 10H2O is calculated as:
CuSO4 → 159.6 g/mol10H2O → 180.16 g/mol (18.016 g/mol × 10)CuSO4 • 10H2O → 159.6 g/mol + 180.16 g/mol
= 339.76 g/mol (rounded to three significant figures)
Thus, we can see that the molar mass of CuSO4 • 10H2O is 339.76 g/mol.
We know that this hydrate consists of ten molecules of water, each having a molar mass of 18.016 g/mol (which is the same as the molar mass of water), and one molecule of CuSO4 with a molar mass of 159.6 g/mol.
Therefore, the number of moles of water in the sample is:
(10 × 18.016 g/mol) ÷ 339.76 g/mol = 0.527 moles
So, the mass of water is equal to its molar mass multiplied by the number of moles.
The mass of water is:
0.527 mol × 18.016 g/mol = 9.49 g
to know more about molecular mass visit:
https://brainly.com/question/15880821
#SPJ11
This tool is used to measure

Answers
Answer:
C. volume
Explanation:
Devise a synthetic sequence for the synthesis of 2,2‑dibromobutane using the list of reagents available. Select the best reagent for each step
Answers
The best reagent for each step is NaNH2, NH2 and CH3I, and HBr.
First of all in the presence of strong base ammonia, we het the clcylated product chain elongation gives but-2-ene followed by bromination gives 2-dibromobutane.
Alkyne using NH3 gets converted into Elongated chain alkyne followed by Hbr.
The reaction of bromine with (E)-stilbene is a classic reaction performed as an example of an electrophilic addition reaction of alkenes. The reaction is stereospecific through an anti addition to the double bond. The traditional reaction uses a bromine solution in methylene chloride, which has very high toxcity.
This bromination reaction demonstrates the stereochemistry of the electrophilic addition of bromine to an alkene. The bromine in this greener approach is generated in situ and eliminates the use of methylene chloride.
To know more about bromination here
https://brainly.com/question/26954199
#SPJ4
In a chemical equation, the left side has the ________ while the right side has the _________.
a) products; reactants b) chemicals; reactants
c) reactants; products d) products; chemicals
Answers
Answer:
c) reactants; products
9. A pebble has a mass of 35 grams and a volume of 14 cubic centimeters.
What is its density? *
Answers
2.5 grams/cubic cm
Explanation:
Density=mass/volume
We can solve for the density of the pebble by using the formula above.
Density=35/14
Density=2.5
I hope this helps! Please comment if you have any questions.
The density of the pebble is 2.5g/cm³
CALCULATE DENSITY:
The density of a substance is its mass in relation to its volume. That is, the density can be calculated by dividing the mass of a substance by its volume.Density = mass (g) ÷ volume (mL)
According to this question, a pebble has a mass of 35 grams and a volume of 14 cubic centimeters (cm³). The density can be calculated as follows:Density = 35g ÷ 14cm³
Density = 2.5g/cm³
Therefore, the density of the pebble is 2.5g/cm³
Learn more: https://brainly.com/question/15164682?referrer=searchResults
How many joules are required to melt 80 grams of ice to water at 0 C?
Answers
Explanation:
Its value is 6.02 kj/mol. This means for every mole of ice we melt we must apply 6.02 kj of heat. We can calculate the heat needed with the following equation:
q
=
n
×
Δ
H
where:
q
= heat
n
= moles
Δ
H
= enthalpy
In this problem we would like to calculate the heat needed to melt 35 grams of ice at 0 °C. This problem can be broken into three steps:
1. Calculate moles of water
2. multiply by the enthalpy of fusion
3. Convert kJ to J
Step 1: Calculating moles of water
35
g
×
(
1
m
o
l
18.02
g
)
=
1.94
m
o
l
s
Step 2: Multiply by enthalpy of fusion
q
=
n
×
Δ
H
=
1.94
×
6.02
=
11.678
k
J
Step 3: Convert kJ to J
11.678
k
J
×
(
1000
J
1
k
J
)
=
11
,
678
J
Finally rounding to 2 sig figs (since 34°C has two sig figs) we get
q
=
12
,
000
J
One last note, if the temperature were not 0 °C then the ice would have to be heated in addition to melted. This would be a phase change problem combined with a heat capacity problem.
Which statement below best describes a catalyst?
Question 3 options:
An item that can slow reactions rates
A molecule that is consumed in a chemical reaction
An item that can increase reaction rates
An item that increases the concentration of reactions
Answers
Please answer question #4
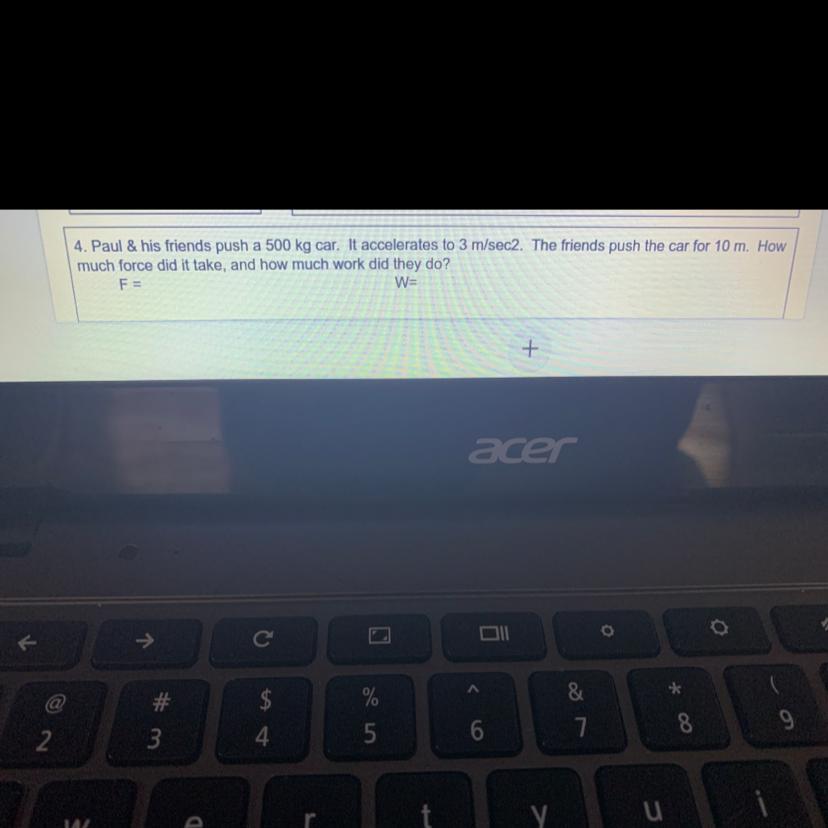
Answers
Answer:
F=500×3 = 1500 N
W = 1500×10 =15000 Nm
The chemical combination of two or more different atoms in fixed amounts is called a(n)
A. element
B. mixture.
C. orbit
D. compound.
Answers
Answer:
D. compound
Explanation:
Answer:
compound
Explanation:
study island
Now suppose a reaction vessel is filled with 0.0406 atm of nitrogen (N_2) and 5.97 atm of ammonia (NH_3) at 1126. Degree C. Answer the following question this system: Under these conditions, will the pressure of N_2 tend to rise or fall? rise fall Is it possible to reverse this tendency by adding H_2? In other words, if you said the pressure of N_2 will tend to rise, can that be changed to a tendency to fall adding H_2? Similarly, if you said the pressure of N_2 will tend to fall, can that be changed to a tendency to rise by adding H_2? Yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of H_2 needed to reverse it. Round your answer to 2 significant digits. atm
Answers
The pressure of \(N_{2}\) will rise under the given conditions. And, Yes, it is possible to reverse this tendency by adding \(H_{2}\). The minimum pressure of H2 required to reverse the tendency is 0.01 atm.
The reaction involved is: \(N_{2}\)(g) + 3\(H_{2}\)(g) ⇌ 2\(NH_{3}\)(g) Hence, when \(H_{2}\) is added to the above system, the \(N_{2}\) and \(H_{2}\) will react to produce \(NH_{3}\). This reaction will reduce the amount of \(N_{2}\) present in the system, causing the pressure of \(N_{2}\) to decrease. Therefore, by adding \(H_{2}\) , we can change the tendency of \(N_{2}\) pressure from rise to fall.To calculate the minimum pressure of \(H_{2}\) required to reverse the tendency, we have to use the equilibrium constant, Kp. The expression for Kp for the above reaction is: Kp =( \(NH_{3}\)) / p(\(N_{2}\)) p3( \(H_{2}\) )
At equilibrium, Kp = 1.7 × 104 at 1126 °C.Now, we will solve for the minimum pressure of \(H_{2}\) needed to reverse the tendency. Let's assume that the pressure of \(N_{2}\) has increased by x atm. Therefore, the new pressure of \(N_{2}\) will be (0.0406 + x) atm. At equilibrium, we have:
p2(\(NH_{3}\) ) / p(\(N_{2}\)) p3( \(H_{2}\) ) = 1.7 × 104
On substituting the given values and simplifying, we get:
p2(\(NH_{3}\)) / p(N2) = 6.39 × 10-5
Now, p2(\(NH_{3}\)) = 5.97 atm, and p(\(N_{2}\)) = (0.0406 + x) atm.
On substituting these values, we get:5.97 / (0.0406 + x) = 6.39 × 10-5
Solving for x, we get:x = 0.00579 atm ≈ 0.01 atm (rounded to 2 significant digits)Therefore, the minimum pressure of \(H_{2}\) required to reverse the tendency is 0.01 atm.
More on equilibrium: https://brainly.com/question/30188799
#SPJ11
Ethanol (1) has a specific heat of 2.443/9-°C and mercury's is 0.14 J/g. C. Which substance is the easier one to warm to a higher temperature? Why?
Answers
Answer:
\( \small \sf Answer \rightarrow Mercury \: is \: easier \: to \: warm. \)
Explanation:
Specific Heat:
The specific Heat of any material is the heat absorbed or evolved by the material to raise or fall it's temperature by 1°C per unit mass of the material.
The heat absorbed or evolved (Q) is directly proportional to the mass of substance(m) and rise or fall of temp(∆T)
The general formula for specific heat is,
\( \sf \: \: \: \: \: s = \frac{Q}{\Delta T} \)
Where s is specific heat,Q is the Heat absorbed or evolved & ∆T is change is temp.Now coming to your question,
The specific heat of ethanol is 2.443 J/g °C
& specific heat of mercury is 0.14 J/g ° C
The definition states that, if the specific heat would be more, more amount of heat will be required for per unit change in temperature and the corresponding substance will be difficult to warm. Similarly, substance with high specific heat won't warm as fast as substance with less specific heat.
Hence mercury will be easier to warm up compared to ethanol, and this could a reason to why Mercury is the only metal liquid at room temperature, because it have less specific heat!
\( \small \sf Answer \rightarrow Mercury \: is \: easier \: to \: warm \: to \: a \: high \: temperature.\)
Thanks for joining brainly community!
write the relation between wavelength frequency and velocity for a sound wave
Answers
Explanation:
frequency; the no of round completed in one second is called frequencywavelength; the distance between two identical point on wave line velocity; the distance cover in one second is called velocityHow many grams are in 4.5 mol of CO2?
Answers
Answer: 44.0095 grams.
Explanation: The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles CO2,
CO(g) + 3 H2(g) → CH4(k) + H2O(g) + heat
The sign of AH is
The sign of AS is
The sign of AG is negative a low or high temperature?
Answers
Explanation:
AH is negative because the reaction gives out heat (exothermic).
AS is negative because the total entropy of the reactants is greater than that of the products (there are 4 moles of gas on reactant side compared to only 1 mole on the product side). Thus, the reactants are said to be "messier" than the products.
AG is negative at lower temperatures because at low temperatures, -TAS is more negative, resulting in the negative sign of AG.
Hope this helps.
When investigating whether or not the substance dibromoethane (ethylene dibro- mide) is carcinogenic, we follow the survival history of 161 white employees of 2 factories who were exposed to dibromoethane. Among them, we observe 7 can- cer deaths in the period 1940-1975. On the other hand, the mean number of cases over that period in that general population is expected to be 5.8. Do those 7 cases provide a reason to consider the substance as carcinogenic?
Answers
When investigating whether or not the substance dibromoethane (ethylene dibromide) is carcinogenic, we follow the survival history of 161 white employees of 2 factories who were exposed to dibromoethane.
Among them, we observe 7 cancer deaths in the period 1940-1975. On the other hand, the mean number of cases over that period in that general population is expected to be 5.8. Do those 7 cases provide a reason to consider the substance as .Yes, the seven cases provide a reason to consider the substance dibromoethane as carcinogenic.
Since the number of cancer deaths observed in the 161 white employees exposed to dibromoethane is 7 which is greater than the expected cancer deaths in the general population, which is 5.8. Therefore, the excess cases may suggest that dibromoethane has some carcinogenic potential.
Hence, we can consider dibromoethane as carcinogenic.
To know more about carcinogenic visit:
https://brainly.com/question/30763696
#SPJ11
what mass of al is required to completely react with 30.0 g mno2 ?what mass of is required to completely react with 30.0 ?12.4 g al 7.76 g al 5.82 g al 10.3 g al
Answers
10.3 g of Al is required to completely react with 30.0 g of MnO₂. The correct answer is option D.
Manganese dioxide reacts with aluminum to produce manganese and aluminum oxide. Here's the balanced chemical equation: 3MnO₂ + 4Al → 3Mn + 2Al₂O₃. Now, let's calculate the mass of Al required to react completely with 30.0 g of MnO₂: From the balanced equation, we can see that 3 moles of MnO₂ react with 4 moles of Al.
The molar mass of MnO₂ is 86.94 g/mol. 30.0 g of MnO₂ is equal to:30.0 g / 86.94 g/mol = 0.3444 mol MnO₂. According to the balanced equation, 0.3444 mol of MnO₂ requires: 4/3 × 0.3444 mol = 0.4592 mol of Al. The molar mass of Al is 26.98 g/mol. 0.4592 mol of Al is equal to: 0.4592 mol × 26.98 g/mol = 12.4 g of Al. Therefore, 12.4 g of Al is required to completely react with 30.0 g of MnO₂. Hence, option D is the correct answer.
Learn more about moles here:
https://brainly.com/question/28239680
#SPJ11
what is the percent yield if the combustion of gasoline results in 680 grams of steam produced when a theoretical yield is 985 grams of steam?
Answers
Answer:
69%
Explanation:
Percent yield = ?
Theoretical yield = 985 grams
Experimental Yield = 680 grams
Percent Yield = Experimental Yield / Theoretical Yield * 100
Percent Yield = 680 / 985 * 1 00
Percent Yield = 0.690 * 100 = 69%
BRAINLIEST
Bridgette wants to conducts science experiment using copper (Cu), salt (NaCl), and vinegar (C₂H₄O₂). Of these, only copper is an element. Why is this true?
Copper is the only material consisting of a single pure substance
Copper is the only material found in nature
Copper is the only material that is a solid
Copper is the only material composed of more than one pure substance
Answers
Answer:
copper is the only material composed of a single pure substance
Explanation:
plss mark brainliest
Answer:
1st opt.
(copper is the only material consisting of a single pure substance)
Explanation:
an element is made up of only one type of atom, and copper (Cu) as you can see is a single pure atom whereas salt (NaCl) and vinegar (C2H4O2) are both made of different elements.
Hope this helps