Answers
Answer:
0.25 mol
Explanation:
Moles= No.of molecules / Avogadros number
Answer:
0.25
Explanation:
Related Questions
An atom has 6 protons, 7 neutrons and 5 electrons. what element is it?
Answers
Answer:
Carbon
Explanation:
Because it was very
1. Fred is walking at 1.7 m/s, he sees a dollar and runs at 2.5 m/s in 1.5 seconds.
What is his acceleration?
Answers
Answer:
Acceleration is the rate of change of velocity. Usually, acceleration means the speed is changing, but not always. When an object moves in a circular path at a constant speed, it is still accelerating, because the direction of its velocity is changing.
Explanation:
It goes with velocity
ellusPerform the followingmathematical operation, andreport the answer to theappropriate number ofsignificant figures.5.1 - 2.3685 =-
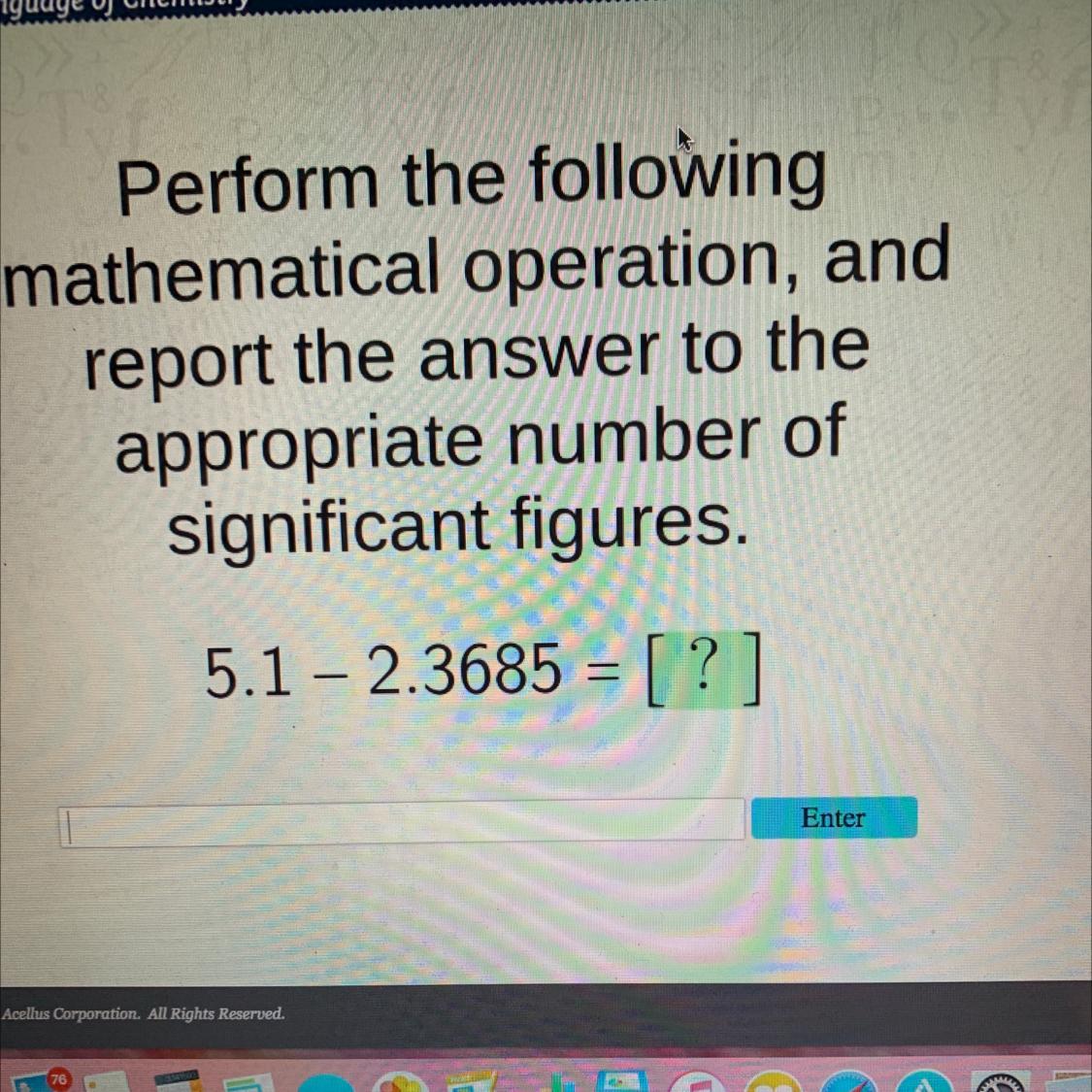
Answers
1) Uncertainty in substraction. the result must have the same number of decimal places as the one with less.
5.1 - 2.3685 = 2.7315
The result must have one decimal place. Since the second decimal place (3) is smaller than five we round down.
The result is 2.7.
Formula Writing Lab for Ionic Compounds Data Table
Answers
The table that can depict the compound is given below
Sodium +1 Na+ Chloride -1 Cl-
Potassium +1 K+ Oxide -2 O2-
Calcium +2 Ca2+ Nitride -3 N3-
Magnesium +2 Mg2+ Sulfide -2 S2-
What are ionic compound?Ionic compounds are the compounds made up of ions that form charged particles when an atom gains or loses electrons. A cation is an ion charged positively; an anion is an ion charged negatively.
In this table, the first column lists the names of the ions, the second column shows their corresponding charges, and the third column displays their ion formulas.
Learn more about compound on
https://brainly.com/question/2687188
#SPJ1
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
Question 7
Anna is rolling a ball down a hill. As the ball rolls, the potential energy is changed to which type of energy?
A chemical energy
B
kinetic energy
С
light energy
D
stored energy
Answers
How much work is done when Bob uses 75 newtons of force to move a block 8 meters?
Answers
Answer:
600 JExplanation:
The work done by an object can be found by using the formula
workdone = force × distance
From the question we have
workdone = 75 × 8
We have the final answer as
600 JHope this helps you
a weather balloon is inflated to a volume 2.2 10square3 L with 374g of helium. what is the density of helium in grams per liter
Answers
Answer:
Density = 0.17 g/L
Explanation:
It is given that,
Volume of the inflated balloon filled with Helium, \(V=2.2\times 10^3\ L\)
Mass, m = 374 g
We need to find the density of helium. It is equal to its mass per unit volume. It can be given by :
d =m/V
\(d=\dfrac{374\ g}{2.2\times 10^3\ L}\\\\=0.17\ g/L\)
So, the density of helium in the balloon is 0.17 g/L.
How many molecules of malic acid (C4H6O5), an acid found in apples and other fruits, are there in 1.25 g
Answers
Answer:
5.61 * 10²¹ molecules
Explanation:
You have 1.25 g of malic acid, C4H.Os, an acid found in apples and other fruits. How many molecules of the acid do you have?
Solution:
A molecule is the group of two or more atoms held together by chemical bonds.
The number of molecules is calculated using the formula:
number of molecules in a substance = number of moles of substance * Avogadro constant
Avogadro constant = 6.02 * 10²³ mol⁻¹
number of moles = mass / molar mass
Given that mass = 1.25 g
molar mass of malic acid (C₄H₆O₅) = (12 * 4) + (1 * 6) + (16 * 5) = 48 + 6 + 80 = 134 g/mol
number of moles = mass / molar mass = 1.25 g / 134 g/mol = 0.0093 mol
Recall that: number of molecules in a substance = number of moles of substance * Avogadro constant
number of molecules in a substance = 0.0093 mol * 6.02 * 10²³ mol⁻¹ = 5.61 * 10²¹ molecules
ILL GIVE BRAINLY PLEASE HELP!!! What type of transport across the cell (plasma) membrane requires energy?
active transport
bilayer
passive transport
concentration gradient
Answers
Active transport requires energy to transport the molecules across the cell membrane. Thus, Option A is correct.
Active transport is the transport of molecules from a lower concentration to a higher concentration across the cell (plasma) membrane. As this process is against the concentration gradient, it requires cellular energy to transport the molecules or ions. Active transport involves Primary active transport and secondary active transport.
Passive transport involves the movement of molecules from a higher to lower concentration gradient and thus does not require energy and is slower than active transport.
Therefore, only active transport requires energy for the transportation of molecules across the cell membrane.
To learn more about active transport,
brainly.com/question/12133248
All of the different types of electromagnetic radiation (light, x-rays, ultraviolet
radiation, and so on) make up the
atomic spectrum
electromagnetic spectrum.
sunlight
spectral lines,
Answers
Answer:
bleh
Explanation:
Pls help i have 0 clue what this even means
Show all work including units and the equation you used to solve. Carbon dioxide gas has a molar mass of 44 g/mol. At 300K and 1.5atm, a sample of carbon dioxide has a volume of 4.5 L. Find the number of moles of the carbon dioxide.
EXTRA POINTS: Find the mass of the carbon dioxide.
Answers
Answer: 0.27 moles of CO2 and 11.88 grams of CO2
Explanation: Use the Ideal Gas Law, PV = nRT, substitute the values given and solve.
I can't seem to upload procedure but:
P = 1.5atm
V = 4.5L
n = moles
R = 0.0821gr/mol (when using atm, kPa is 8.31)
T = 300K
Isolate what you don't have, in this case n. Change PV = nRT to PV/RT = n. Substitute the values to get moles. Once you have this, multiply the value by the molar mass of CO2 (44gr/mol) to get the mass of CO2 in grams.
A doorbell uses an electromagnet for its operation.
Question 7 options:
True
False
Answers
Explanation:
The heart of the doorbell is an electromagnetic so yeah it does.
Electromagnets are coils of wire wrapped around a small piece of magnetic metal. When electricity passes through the wire, it creates a magnetic field around the wire. When you press a doorbell button, you complete an electrical circuit that allows household electricity to flow through the doorbell's internal electromagnet.
What is the change in temperature of 150 grams of water when gains 500 Joules of energy?. The Cp for
water is 4.18 J/gxC
Answers
The change in temperature of 150 grams of water when gains 500 Joules of energy is 0.797 °C
What is Heat Transfer ?Heat transfer is defined as the process in which the molecules are moved from the region of higher temperature to lower temperature.
The heat transfer can be calculated by the formula
\(Q=m \times c \times \Delta T\)
Here, Q is the heat supplied to the system,
m is the mass of the system,
c is the specific heat capacity of the system and
Δ T is the change in temperature of the system.
Substituting the values in the equation
m = 150g
Q=500 Joules
\(\rm c_{p}\) = 4.18 J/gxC
500 = 150 * 4.18* Δ T
Δ T = 0.797 °C
Therefore the change in temperature of 150 grams of water when gains 500 Joules of energy is 0.797 °C .
To know more about Heat Transfer
https://brainly.com/question/13433948
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
How many molecules are in 24 grams of ozone (03)
Answers
Answer:48
Explanation:
Answer: 3. 0.125 X 10”23 molecules
Explanation:
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
What type of radioactive decay is illustrated by the following nuclear equation? N ->0 + e positron emission b. alpha decay beta decay gamma production helium emission wnar nroni Search
Answers
A nuclear equation can be used to represent alpha decay. If the total numbers of protons and neutrons on both sides of the arrow are equal, the equation is balanced.
While all radioactive decay poses a threat to life, alpha decay poses the least threat. beta decay The transformation of the uranium-238 nucleus into the thorium-234 nucleus is an illustration of this decay. Half-lives are another unit used to express the rate of nuclear decay. A particular isotope's half-life is the amount of time it takes for its radioactivity to decay by half. A radioisotope having a half-life of 14 days will have had half of its atoms decay within that time frame. For instance, after generating intermediates like Uranium-234, Thorium-230, and Lead-206, the decay chain that starts with Uranium-238 ends in Lead-206.
To learn more about radioactive the given link:
https://brainly.com/question/1236735
#SPJ4
A 5.0g sample of MgCl2 may contain measurable amounts of other compounds as impurities. Which of the following quantities is (are) needed to determine that the sample is pure MgCl2 ?
Answers
Answer:
The mass of Mg and the mass of Cl in the sample
Explanation:
We have that the sample's is purity is simply the mass of Mg and the mass of Cl in the sample
From the Question we are told that
Mass of MgCl2 =5g
Therefore
What we need to determine that the sample's is purity is simply the mass of Mg and the mass of Cl in the sample because
the mass of MgCl should be equal to mass of Mg and mas of Cl
For more information on this visit
https://brainly.com/question/17391424
What ideas do you have about why Christchurch’s air temperature is cooler during el niño years?
Answers
One possible reason for cooler air temperatures in Christchurch during El Niño years is the shift in atmospheric circulation patterns.
During El Niño years, Christchurch may experience cooler air temperatures due to several factors associated with the El Niño phenomenon.
El Niño is characterized by the abnormal warming of the surface waters in the eastern tropical Pacific Ocean, which has global climatic implications. While El Niño is primarily associated with changes in oceanic conditions, its effects can extend to atmospheric patterns, leading to altered weather patterns and temperature variations.
One possible reason for cooler air temperatures in Christchurch during El Niño years is the shift in atmospheric circulation patterns. El Niño can disrupt the normal global atmospheric circulation, resulting in changes in the positioning and intensity of weather systems.
This can lead to the advection of cooler air masses from the south or southeast towards Christchurch, resulting in cooler temperatures.
Another factor is the influence of El Niño on regional rainfall patterns. El Niño often leads to drier conditions in the South Island of New Zealand, including Christchurch.
Reduced cloud cover and less moisture in the air can contribute to cooler temperatures as there is less insulation from the sun's radiation and less evaporative cooling. Additionally, the absence of significant rainfall can result in less moisture in the soil, leading to cooler conditions as less energy is used for evaporation.
For more such questions on temperatures visit:
https://brainly.com/question/4735135
#SPJ8
For each of these pairs of half-reactions, write the balanced equation for the overall cell reaction and calculate the standard cell potential. Express the reaction using cell notation. You may wish to refer to Chapter 20 to review writing and balancing redox equations.
1.
Pt2+(aq)+2e-Pt(s)
Sn2+(aq)+2e-Sn(s)
2.
Co2+(aq)+2e-Co(s)
Cr3+(aq)+3e-Cr (s)
3.
Hg2+(aq)+2e-Hg (I)
Cr2+(aq)+2e-Cr (s)
please help out
Answers
1. For the pair of half-reactions:
Pt2+(aq) + 2e- → Pt(s) ... (1)
Sn2+(aq) + 2e- → Sn(s) ... (2)
To obtain the balanced equation for the overall cell reaction, we need to multiply the half-reactions by appropriate coefficients to ensure that the number of electrons transferred is equal. In this case, we can multiply equation (1) by 2 and equation (2) by 1:
2(Pt2+(aq) + 2e-) → 2(Pt(s))
Sn2+(aq) + 2e- → Sn(s)
Combining the equations, we have:
2Pt2+(aq) + Sn2+(aq) → 2Pt(s) + Sn(s)
The cell notation for this reaction is:
Pt2+(aq) | Pt(s) || Sn2+(aq) | Sn(s)
To calculate the standard cell potential (E°), we need to know the standard reduction potentials for Pt2+/Pt(s) and Sn2+/Sn(s) half-reactions. Referring to standard reduction potential tables, we find:
E°(Pt2+/Pt(s)) = +1.20 V
E°(Sn2+/Sn(s)) = -0.14 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = 0.00 V - (-0.14 V) = +0.14 V
Therefore, the standard cell potential for this reaction is +0.14 V.
2. For the pair of half-reactions:
Co2+(aq) + 2e- → Co(s) ... (3)
Cr3+(aq) + 3e- → Cr(s) ... (4)
To balance the number of electrons transferred, equation (4) can be multiplied by 2:
2(Co2+(aq) + 2e-) → 2(Co(s))
Cr3+(aq) + 3e- → Cr(s)
Combining the equations, we have:
2Co2+(aq) + Cr3+(aq) → 2Co(s) + Cr(s)
The cell notation for this reaction is:
Co2+(aq) | Co(s) || Cr3+(aq) | Cr(s)
To calculate the standard cell potential (E°), we refer to the standard reduction potentials:
E°(Co2+/Co(s)) = -0.28 V
E°(Cr3+/Cr(s)) = -0.74 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = -0.74 V - (-0.28 V) = -0.46 V
Therefore, the standard cell potential for this reaction is -0.46 V.
3. For the pair of half-reactions:
Hg2+(aq) + 2e- → Hg (l) ... (5)
Cr2+(aq) + 2e- → Cr(s) ... (6)
The equation for the overall cell reaction can be obtained by multiplying equation (6) by 2:
2(Hg2+(aq) + 2e-) → 2(Hg (l))
Cr2+(aq) + 2e- → Cr(s)
Combining the equations, we have:
2Hg2+(aq) + Cr2+(aq) → 2Hg (l) + Cr(s)
For more such questions on balanced equation.
https://brainly.com/question/11904811
#SPJ8
actetic acid only partially ionizes in water
Answers
Acetic acid only partially ionizes in water as it is a weak acid.
Weak Acids are the acids that do not completely dissociate into their constituent ions when dissolved in solutions.
When dissolved in water, an equilibrium is established between the concentration of the weak acid and its constituent ions.
Acetic acid, also known as ethanoic acid, is a weak acid with the chemical formula CH₃COOH. It is known to be the active component of vinegar.
Learn more about Weak acids, here:
https://brainly.com/question/22104949
#SPJ1
. What mass of ammonium chloride must be added to 250. mL of water to give a solution with pH
4.85? [Kb(NH3) = 1.8 x 10-1
a. 4.7 g
b. 75 g
c.2.3 x 10-ºg
d. 19 g
e.10g
Answers
The mass of ammonium chloride that must be added is : ( A ) 4.7 g
Given data :
Volume of water ( V ) = 250 mL = 0.25 L
pH of solution = 4.85
Kb = 1.8 * 10⁻⁵
Kw = 10⁻¹⁴
Given that the dissolution of NH₄Cl gives NH₄⁺⁺ and Cl⁻ ions the equation is written as :
NH₄CI + H₂O ⇄ NH₃ + H₃O⁺
where conc of H₃O⁺
[ H₃O⁺ ] = \(\sqrt{Ka.C}\) and Ka = Kw / Kb
∴ Ka = 5.56 * 10⁻¹⁰
Next step : Determine the concentration of H₃O⁺ in the solution
pH = - log [ H₃O⁺ ] = 4.85
∴ [ H₃O⁺ ] in the solution = 1.14125 * 10⁻⁵
Next step : Determine the concentration of NH₄CI in the solution
C = [ H₃O⁺ ]² / Ka
= ( 1.14125 * 10⁻⁵ )² / 5.56 * 10⁻¹⁰
= 0.359 mol / L
Determine the number of moles of NH₄CI in the solution
n = C . V
= 0.359 mol / L * 0.25 L = 0.08979 mole
Final step : determine the mass of ammonium chloride that must be added to 250 mL
mass = n * molar mass
= 0.08979 * 53.5 g/mol
= 4.80 g ≈ 4.7 grams
Therefore we can conclude that the mass of ammonium chloride that must be added is 4.7 g
Learn more about ammonium chloride : https://brainly.com/question/13050932
why is coal and fossil fuels a nonrenewable resource
Answers
Answer:
It is a finite resource. Fossil fuels such as oil, natural gas, and coal are examples of nonrenewable resources. Humans constantly draw on the reserves of these substances while the formation of new supplies takes eons. Renewable resources are the opposite: Their supply replenishes naturally or can be sustained.
Explanation:
What is the molar mass
MgCrO4
Answers
The molar mass of MgCrO4 is approximately 140.30 g/mol.
To determine the molar mass of MgCrO4 (magnesium chromate), we need to calculate the sum of the atomic masses of each individual element in the compound.
The chemical formula MgCrO4 indicates that the compound consists of one magnesium atom (Mg), one chromium atom (Cr), and four oxygen atoms (O).
The atomic masses of the elements can be found on the periodic table:
Magnesium (Mg) has an atomic mass of approximately 24.31 g/mol.
Chromium (Cr) has an atomic mass of around 51.99 g/mol.
Oxygen (O) has an atomic mass of about 16.00 g/mol.
Now, we can calculate the molar mass of MgCrO4 by summing up the atomic masses of each element, considering the respective subscripts:
Molar mass = (Atomic mass of Mg) + (Atomic mass of Cr) + 4 × (Atomic mass of O)
Molar mass = (24.31 g/mol) + (51.99 g/mol) + 4 × (16.00 g/mol)
Molar mass = 24.31 g/mol + 51.99 g/mol + 64.00 g/mol
Molar mass ≈ 140.30 g/mol
for more such questions on mass
https://brainly.com/question/24191825
#SPJ8
Cooking Oil
Specific Heat (J/g°C)
Corn oil
2.50
Olive oil
1.96
Sesame oil
1.63
Soybean oil
1.97
Vegetable oil
1.67
Based on the table above,
___ oil would
transfer heat the best because its specific heat is
the _____
Answers
Answer:
Sesame oil
lowest
Explanation:
bruh
Based on the table provided, corn oil would transfer heat the best because its specific heat is the highest.
Specific heat is a measure of the amount of heat energy required to raise the temperature of a substance by a certain amount. A higher specific heat value indicates that the substance can absorb more heat energy without a significant increase in temperature.
In the given table, corn oil has the highest specific heat value of 2.50 J/g°C. This means that corn oil can absorb a relatively larger amount of heat energy per gram compared to the other oils listed. Consequently, corn oil would be more effective in transferring heat compared to the other oils listed, as it can store and release more heat energy per unit mass.
Learn more about Specific heat from the link given below.
https://brainly.com/question/31608647
#SPJ2
What is the molar mass of copper 1 carbonate when rounding to the nearest whole number of copper 56 and carbon 12 and oxygen 16
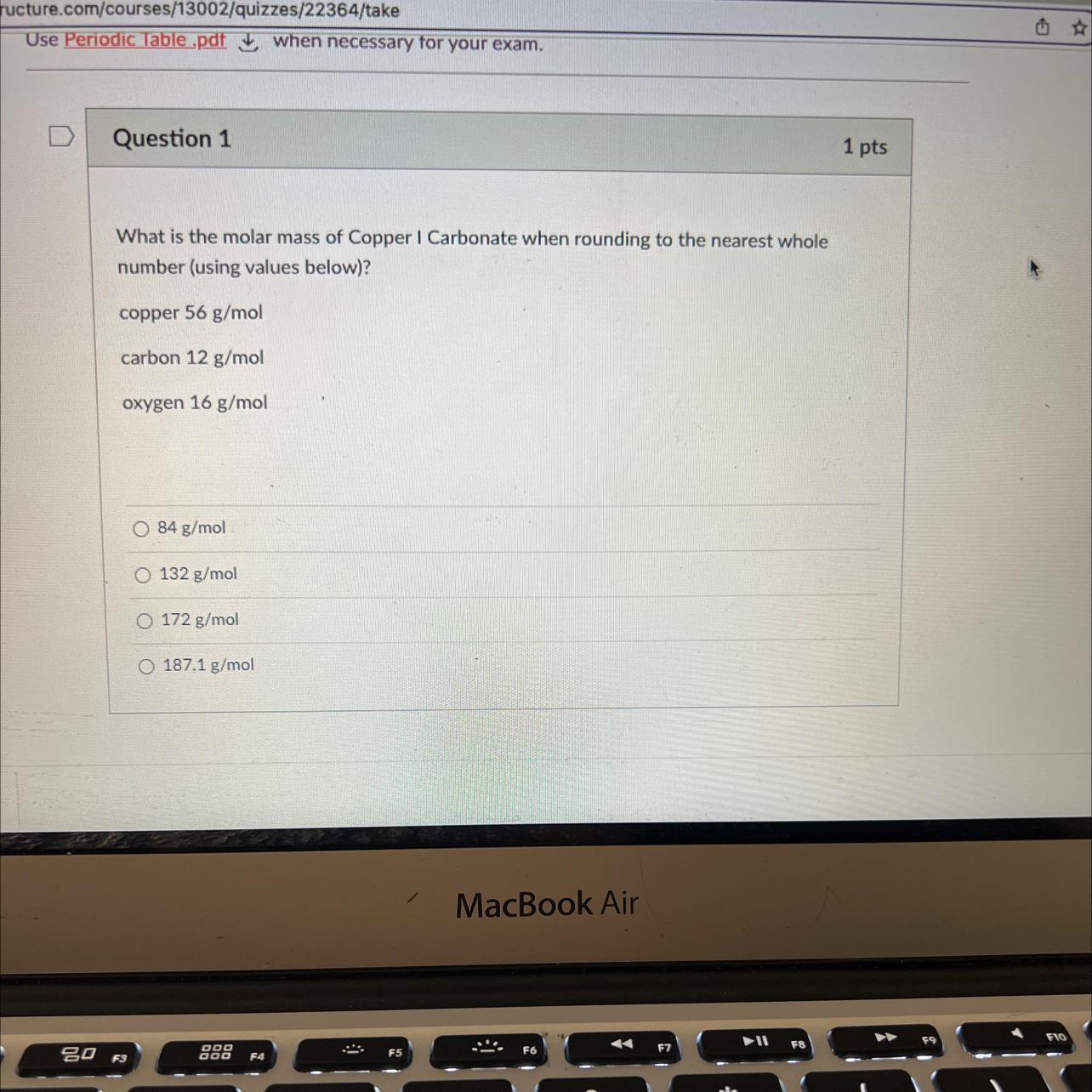
Answers
Answer
172 g/mol
Explanation
The chemical formula of copper I carbonate is Cu₂CO₃
Using the given molar masses of:
Cu = 56 g/mol
C = 12 g/mol
O = 16 g/mol
The molar mass of Cu₂CO₃ = (2 x 56 g/mol) + 12 g/mol + (3 x 16 g/mol)
Cu₂CO₃ = 112 g/mol + 12 g/mol + 48 g/mol
Cu₂CO₃ = 172 g/mol
The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
.
According to the octet rule, this element would be expected to form an ion with a charge of
.
If is in period 5, the ion formed has the same electron configuration as the noble gas
.
The symbol for the ion is
.
Answers
This element is in group 1.
According to the octet rule, this element would be expected to form an ion with a charge of +1.
If X is in period 5, the ion formed has the same electron configuration as the noble gas Krypton
The symbol for the ion is Rb⁺
What is electronic configuration?Electronic configuration refers to the arrangement of electrons in the orbitals of an atom or molecule, indicating the energy level of the electrons, the number of electrons in each energy level, and the number of electrons in each orbital.
Considering the given element:
It has one valence electron, hence it is in group 1. Group 1 elements form ions with a charge of +1.
Losing one electron will give the ion the same electron configuration as Kyrton since it is the noble gas in Period 4.
The element is rubidium and the ion is Rb⁺.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1

Select the structure that corresponds
to the name:
3,5,6-trichloro-2-heptanol

Answers
Answer:C both
Explanation:
The Structure that correspondsto the name: 3,5,6-trichloro-2-heptanol is C both.
How nomenclature is done in organic compound ? First name the principal carbon chain and also check type of bonds single or double present on the carbon chain. Name the functional group according to Seniority table. Name the substituent alphabetically. Both are isomers∵ all are substituent and OH alcohol is added on the principal carbon chain both names are suggested.
Learn more about nomenclature of organic compound here: http://brainly.brainly.com/question/1594044
#SPJ2
Predict the products of the following reaction:
HCN + RbOH
Answers
Answer:
Rubidium Cyanide and Water
Explanation: