Answers
Answer:
35.3 g
Explanation:
Step 1: Given data
Number of atoms of aluminum: 7.90 × 10²³ atoms
Step 2: Calculate the number of moles corresponding to 7.90 × 10²³ atoms of aluminum
We will use Avogadro's number: there are 6.02 × 10²³ atoms of aluminum in 1 mole of atoms of aluminum.
7.90 × 10²³ atoms × (1 mol/6.02 × 10²³ atoms) = 1.31 mol
Step 3: Calculate the mass corresponding to 1.31 moles of aluminum
The molar mass of Al is 26.98 g/mol.
1.31 mol × 26.98 g/mol = 35.3 g
Related Questions
How many mg does a 643 kg sample contain?
Answers
Answer:
\(6.43x19^8mg\)
Explanation:
Hello,
In this case, this unit conversion is performed by knowing that 1 kg of mass contains 1000 g and 1 g contains 1000 mg, therefore, the result is:
\(643kg*\frac{1000g}{1kg} *\frac{1000mg}{1g}\\ \\=6.43x10^8mg\)
Regards.
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
If 18*10`23 molecules of H2 react with nitrogen, how many moles of NH3 are produced
Answers
Answer:
0.2moles
Explanation:
Given parameters:
Number of molecules of H₂ = 1.8 x 10²³molecules
Unknown:
Number of moles of NH₃ = ?
Solution:
The reaction equation is given as:
N₂ + 3H₂ → 2NH₃
Let us solve from the known to the unknown specie;
Since the number of molecules is 1.8 x 10²³molecules of H₂;
6.02 x 10²³molecules will give 1 mole of a substance
1.8 x 10²³molecules will produce \(\frac{1.8 x 10^{23} }{6.02 x 10^{23} }\) mole of H₂
= 0.3moles of H₂
From the balanced chemical equation;
3 moles of H₂ produced 2 moles of NH₃
0.3 moles of H₂ will yield \(\frac{0.3 x 2}{3}\) = 0.2moles
how many atoms are in 5 moles of chromium
Answers
Given:
- Moles of chromium (Cr): 5 moles
Number of atoms = Moles of substance x Avogadro's number
Number of atoms = 5 moles x (6.022 x 10^23 atoms/mole)
Number of atoms = 3.011 x 10^24 atoms
Therefore, there are approximately 3.011 x 10^24 atoms in 5 moles of chromium.
which is a way to express concentration of a solution? parts per billion moles molar mass force per square meter
Answers
Answer:
parts per billion
Explanation:
I took the quiz
Answer:
parts per billion
Explanation:
edge 2021
What is the density of Ar(g) at -11°C and 675 mmHg?
Answers
Answer:
The Density Of Ar (g) At -11°C And 675 MmHg (R =0.08206 L·atm/mol·K, 1 Atm = 760mmHg).
Cisplatin Pt(NH3)2Cl2 has been studied and used as an anti-tumor agent to treat certain types of cancer. The compound is generated by the following reaction:K2PtCl4 2NH3 -> Pt(NH3)2Cl2 2KCl A pharmacy supplier combines 655.1 Kg K2PtCl4 with 728 Kg NH3 to make cis-plat for an order from a hospital chain. a. Determine the limiting and excess reagent.b. What is the maximum amount of cis-plat produced
Answers
Answer:
* Limiting reactant: K2PtCl4.
* \(m_{Pt(NH_3)_2Cl_2}=475kgPt(NH_3)_2Cl_2\)
Explanation:
Hello,
In this case, for the reaction:
\(K_2PtCl_4 +2NH_3 \rightarrow Pt(NH_3)_2Cl_2 +2KCl\)
By starting with 655.1 kg of K2PtCl4 (molar mass 415.1 g/mol) and 728 kg of NH3 (molar mass 17 g/mol) the limiting reactant is identified as the one yielding the smallest moles of cisplatin, thus, we proceed as follows:
\(n_{Pt(NH_3)_2Cl_2}^{by \ K_2PtCl_4}=655.1kg K_2PtCl_4*\frac{1kmol K_2PtCl_4}{415.1g K_2PtCl_4}*\frac{1molPt(NH_3)_2Cl_2}{1mol K_2PtCl_4} =1.578kmolPt(NH_3)_2Cl_2\\\\n_{Pt(NH_3)_2Cl_2}^{by \ NH_3}=728kgNH_3*\frac{1kmol NH_3}{17g NH_3}*\frac{1molPt(NH_3)_2Cl_2}{2molNH_3} =21.4kmolPt(NH_3)_2Cl_2\)
In such a way, we infer that the K2PtCl4 is the limiting reactant, therefore, the maximum amount of cis-plat (300 g/mol) produced is:
\(m_{Pt(NH_3)_2Cl_2 }=1.578kmolPt(NH_3)_2Cl_2*\frac{301gPt(NH_3)_2Cl_2}{1molPt(NH_3)_2Cl_2}\\ \\m_{Pt(NH_3)_2Cl_2}=475kgPt(NH_3)_2Cl_2\)
Best regards.
Based on the synthesis reaction, what would the product of the reaction be? NaPO3 + CuO → ? NaO + CuPO3 Na + P + Cu + O4 NaCuPO4 NaCu + PO4
Answers
Answer:
C
Explanation:
Based on the synthesis reaction,the product of the given chemical equation is NaCuPO4 .
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ2
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). If you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 392 mmHg, what is the pressure (in mmHg) of water vapor after the reaction has completed (temperature and volume do not change).
Answers
Answer:
118.776 mmHg
Explanation:
The equation of the reaction is;
C4H10(g) + 13/2 O2(g) ------> 4CO2(g) + 5H20(g)
Now the mole ratio according to the balanced reaction equation is;
1 : 6.5 : 4 : 5
Hence, the total number of moles present = 1 + 6.5 + 4 + 5 = 16.5 moles
Mole fraction of water vapour = 5/16.5 = 0.303
We also know that;
Partial pressure= mole fraction * total pressure
Partial pressure of H20(g) = 0.303 * 392 mmHg = 118.776 mmHg
The pressure (in mmHg) of water vapor is 118.78 mmHg
Balanced equation for the reaction
Butane reacts with oxygen according to the following equation
2C₄H₁₀ + 13O₂ —> 8CO₂ + 10H₂O
How to determine the mole fraction of water Mole of butane = 2 moles Mole of oxygen = 13 molesCarbon (IV) oxide = 8 moles Mole of water = 10 moles Total moles = 2 + 13 + 8 + 10 = 33 moles Mole fraction of water =?Mole fraction = mole / total mole
Mole fraction of water = 10 / 33
Mole fraction of water = 0.303
How to determine the partial pressure of waterMole fraction of water = 0.303Total pressure = 392 mmHgPartial pressure of water =?Partial pressure = mole fraction × total pressure
Partial pressure of water = 0.303 × 392
Partial pressure of water = 118.78 mmHg
Learn more about partial pressure:
https://brainly.com/question/15577259
A system releases 481 kJ of heat while 390 kJ of work is done on the system. What is the change in internal
energy?
Answers
Answer:
ΔE = q + w
here q = -136 kj
w = 146
so ΔE = -136 + 146 = 10 kj
thus internal energy of system increases by 10 kj...
I hope this helps! :) I don't know if it's the right one
When a strong acid or base is added to water it...
Answers
When a strong acid or base is added to water, the pH will change dramatically.
Strong AcidA strong acid is one that is completely dissociated or ionized in an aqueous solution. This means it gives off the greatest number of hydrogen ions or protons when placed in a solution. Examples of strong acid are HCl, HBr, H2SO4, HNO4. These acids when placed in water, produces greatest amount of hydrogen ions. The pH value changes drastically. Any that has very high concentration of hydrogen and ion is acidic.
Also when base is added to water, the pH of water will increase above 7 and become basic. The pH of water is 7, but when base is added to it increases above 7.
Base is any solution that is slippery to touch in water solution, changes color, react with acid to form salt and change red litmus paper to blue.
Learn about acid and base in water solution here
https://brainly.com/question/27915098
#SPJ1
Write the solubility product expressions for the following compounds.
(a) Ag2CO3
(b) Hg2Cl2
Answers
(a) The solubility product expression for Ag2CO3 is:
Ksp = [Ag+]^2[CO3^2-]
(b) The solubility product expression for Hg2Cl2 is:
Ksp = [Hg2^2+][Cl^-]^2
Need answers for Chemistry asap. Will give brainliest.

Answers
Answer:
Balance chemical equation is
O2(g) + 2H2(g) --> 2H20(g)
a. 0.20 mol 02 --> 0.225mol H20
b. 0.30 mol H2 --> 2.7mol H20
Answer:
a) 0.4 moles
b) 0.3 moles

Aqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H₂O).
Suppose 55.8 g of hydrobromic acid is mixed with 15. g of sodium hydroxide. Calculate the maximum mass of sodium bromide that could be produced by the
chemical reaction. Be sure your answer has the correct number of significant digits.
Answers
The maximum mass of sodium bromide that could be produced by the chemical reaction is 38.59 g.
Steps
The balanced chemical equation for the reaction between hydrobromic acid and sodium hydroxide is:
HBr (aq) + NaOH (s) → NaBr (aq) + H2O (l)
To determine the maximum mass of sodium bromide that could be produced, we need to first identify the limiting reactant. We can do this by calculating the number of moles of each reactant using their molar masses:
Molar mass of HBr = 80.91 g/mol
Molar mass of NaOH = 40.00 g/mol
Number of moles of HBr = 55.8 g ÷ 80.91 g/mol = 0.689 mol
Number of moles of NaOH = 15.0 g ÷ 40.00 g/mol = 0.375 mol
The stoichiometry of the balanced chemical equation tells us that one mole of HBr reacts with one mole of NaOH to produce one mole of NaBr. Therefore, the limiting reactant is NaOH, since it produces fewer moles of product (NaBr) than HBr.
The number of moles of NaBr produced from the reaction is equal to the number of moles of NaOH used, which is 0.375 mol.
Finally, we can calculate the maximum mass of NaBr that could be produced using its molar mass:
Molar mass of NaBr = 102.89 g/mol
Mass of NaBr = number of moles of NaBr × molar mass of NaBr
Mass of NaBr = 0.375 mol × 102.89 g/mol = 38.59 g
Therefore, the maximum mass of sodium bromide that could be produced by the chemical reaction is 38.59 g.
learn more about molar mass here
https://brainly.com/question/837939
#SPJ1
If you use a hammer to weather a piece of chalk is it physical or chemical weathering and how do you know?
Answers
What is arranged to form a material with a crystal structure
Answers
Answer:
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. The scientific study of crystals and crystal formation is known as crystallography.
Explanation:
How many moles in 2.33E25 molecules of NO?
0.0258 mol
3.87E20 mol
38.7 mol
2.58E48 mol
please show work
Answers
Answer:
0.0258 mol Answer .......
Why does the atom get more reactive if the nucleus is far away from the outer electron.
Answers
Answer: The size of an atom is generally considered as the distance between the outer shell electrons and the nucleus, i.e., the atom's radius. The larger the atomic radius, the lesser the reactivity of an element. It is because when the electrons are away from the nucleus, there is less electron density on the atom.
chuyển hoá Nh3 - chu trình ure
Answers
Answer:
umm translate
Explanation:
Which of these properties is the best one to use for indentification of an element
Answers
Answer:
you need to state the options
The tomato is dropped. What is the velocity, v
, of the tomato when it hits the ground? Assume 86.0 %
of the work done in Part A is transferred to kinetic energy, E
, by the time the tomato hits the ground.
Express your answer with the appropriate units.
Answers
To determine the tomato's velocity when it hits the ground, we need more information. Specifically, we need the height from which the tomato was dropped and the tomato mass.
Without these details, it is impossible to calculate velocity accurately. The velocity of an object when it hits the ground depends on factors such as the height of the fall, the mass of the object, and any forces acting on it during the fall (such as air resistance).
If you can provide the necessary information, I can help you calculate the velocity of the tomato when it hits the ground.
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
Where does the oil in cars come from?
SUBJECT: AQUATIC SCIENCE
Answers
The oil from cars is gotten from crude oil.
Crude oil and its sources:The car is an automobile that is mainly used for transportation purposes. It is made up of both electrical and mechanical parts.
The mechanical part of the car is driven by the engine which is the heart of the car.
The engine is made up of adjacent moving parts that need to be frequently lubricated through the engine oil.
The engine oil is gotten from crude oil, which is a mixture of hydrocarbons that exist in liquid phase in natural underground reservoirs.
Therefore, the oil from cars is gotten from crude oil.
Learn more about crude oil here:
https://brainly.com/question/14260176
Calculate the Ka of your acetic acid solution. Discuss this calculation. Based on the value of Ka, is acetic acid a strong acid or a weak acid
Answers
Based on our knowledge of strong and weak acids, we can confirm that the Ka value for acetic acid will be relatively low since it is a weak acid.
Acids can be strong or weak. This is determined by its tendency to break apart into ions or stay together to form molecules. Although somewhat counter-intuitive, strong acids are those that are most likely to break apart and therefore contain a high number of ions within their solutions.
Weak acids, on the other hand, are those that tend to stay together in the form of molecules and therefore possess very low ion counts in their solutions. The acid dissociation constant, Kₐ, is used to measure whether an acid is weak or strong and how much so. In the case of Acetic acid, the ka measurement will offer a low value, indicating a weak acid.
To learn more visit:
https://brainly.com/question/4131966?referrer=searchResults

what does celery, a wooden spoon, and oil/gasoline have in common?
Answers
Answer:
All of them are organic compounds which have carbon as their main atom in the structure.
Explanation:
Hello.
In this case, since organic chemistry is the study of all the compounds having the carbon atom as their main atom, all the vegetables, animals, an in general, living things are composed by lipids, proteins, and other organic substances with this feature. Moreover, wood-based materials are mainly composed by lignin which is an organic polymer also having carbon as the main atom. In addition, oil and gasoline are organic chemical compounds with a lot of applications in daily life which also contain carbon atoms in their structure.
In such a way, a celery, a wooden spoon, and oil/gasoline have the carbon atom in common as their main atom in their chemical structures.
Best regards.
Classify the following mixtures as homogeneous or heterogeneous. Drag the appropriate items to their respective bins. View Available Hint(s) Reset Help brass gravel vodka potato salad sugar STUP Homogenous mixture Heterogeneous mixture
Answers
Homogeneous mixture are Brass, Vodka and Sugar; while Heterogeneous mixture are Gravel and Potato salad.
A homogeneous mixture is a type of mixture where the composition is uniform and the same throughout. Examples of homogeneous mixtures include saltwater, air, and vinegar. These mixtures have a consistent appearance and properties, and their individual components cannot be easily separated.
A heterogeneous mixture is a mixture where the composition is not uniform and can vary in different parts of the mixture. Examples of heterogeneous mixtures include salad, fruit punch, and soil. These mixtures have varying appearance and properties, and their individual components can be easily separated.
You can learn more about Homogenous mixture & Heterogeneous mixture at
https://brainly.com/question/14441492
#SPJ4
Which wave has the greatest distance from trough to trough?
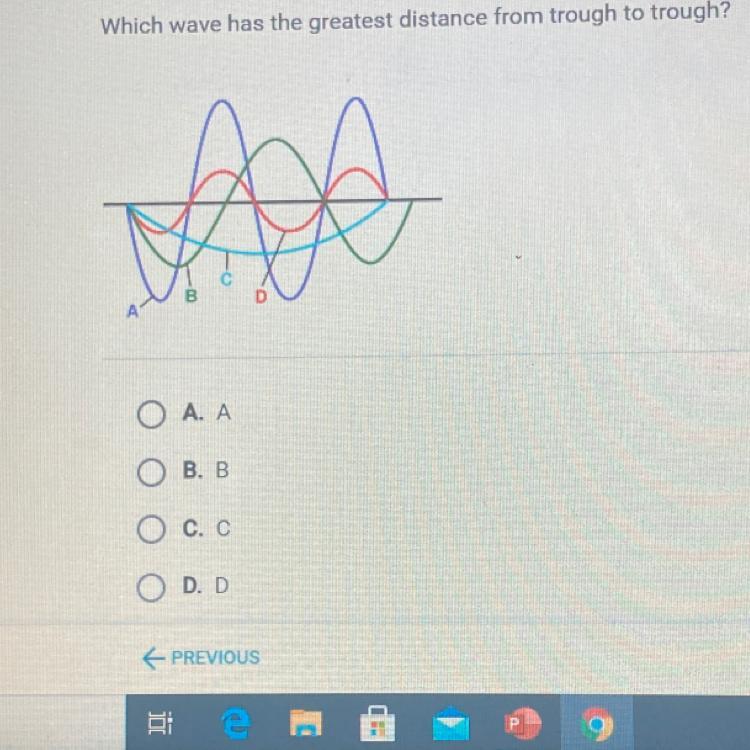
Answers
Answer:
C.C
Explanation:
Just took the test
A transverse wave is a motion in which all points on a wave oscillate along paths at right angles. Hence, option C is correct.
On a wave, what is the distance from trough to trough?The wavelength is the horizontal distance between two adjacent crests or troughs.
The longest distance a particle goes from rest is to the peak of a crest or the bottom of a trough in a transverse wave. The amplitude of a transverse wave is this distance.
Hence, option C is correct.
Learn more about wavelength here:
https://brainly.com/question/13533093
#SPJ2
A 2.550×10−2 M glycerol solution (C3H8O3) in water is at 20.0 ∘C . The sample was created by dissolving a sample of C3H8O3 in water and then bringing the volume up to 1.000 L. It was determined that the volume of water needed to do this was 998.9 mL. The density of water at 20.0 ∘C is 0.9982 g/mL
a.) Calculate the molality of the glycerol solution
b.) Calculate the mole fraction of glycerol in this solution
c.) Calculate the concentration of the glycerol solution in percent by mass
d.) Calculate the concentration of the glycerol solution in parts per million by mass
Answers
The molality of the solution is 0.0256 m.
The mole fraction of glycerol is 0.00046
The percent by mass concentration of glycerol is 0.23%
The ppm concentration is 2300 ppm
What is the molality?Molality is a measure of the concentration of a solute in a solution. It is defined as the number of moles of solute per kilogram of solvent.
The formula for molality is:
molality = moles of solute / mass of solvent in kilograms
1) Density of water = mass/volume
Mass of water = Density * volume of water
Mass =\(0.9982 g/mL * 998.9 mL\)
Mass =0.997 Kg of water
Number of moles of the glycerol = \(2.550* 10^-2 M * 1 L\)
= \(2.550*10^-2\) moles
Molality of the solution = \(2.550*10^-2\) moles/0.997 Kg
= 0.0256 m
Number of moles of water = 998.9/18 g/mol
= 55.5 mole
Mole fraction of glycerol = \(2.550*10^-2\) /\(2.550*10^-2\) + 55.5
= 0.00046
By percent by mass;
2.3/1001.2 * 100/1
= 0.23%
Mass of glycerol = 2.3 g
Volume of solution = 1 L
Thus we have concentration in ppm as;
\(2.3 * 10^3\) mg/ 1 L =2300 ppm
Learn more about molality:https://brainly.com/question/26921570
#SPJ1
Balancing equations, Lawd help me

Answers
Every chemical has an equal amount of atoms on both the product and reactant sides, and the equation is considered to be balanced without any inequalities. Hence, C3H8 + 5O2 3CO2 + 4H2O is the balanced chemical equation.
How is an equation balanced?In order to make a stoichiometry equal in both the reactants and the products, add equations whenever necessary prior to the symbols or formulations.
What is the balancing rule?In science, if an element is still, we state it is balanced. An thing is in a thermodynamic equilibrium when it's fully balanced. Any forces acting on the item are counterbalanced by forces moving the other way. The average location of the gravitational pull on an item is known as the centre of gravity.
To know more about chemical equation visit:
https://brainly.com/question/30087623
#SPJ1
what component separate the dissolved salts?
Answers
Answer:
You could seperate dissolved salt by the process of Distillation
Explanation:
Simple distillation is a method for separating the solvent from a solution. For example, water can be separated from salt solution by simple distillation. This method works because water has a much lower boiling point than salt. When the solution is heated, the water evaporates.