A peptide bond is formed between two amino acids by the reaction of the ______ of one amino acid with the ______ of the other.
Answers
A peptide bond is formed between two amino acids by the reaction of the α-carboxyl group of one amino acid with the α-amino group of the other amino acid.
A peptide is a chain of amino acids that is attached to another amino acid by a peptide bond (and sometimes by an isopeptide bond). Organisms use enzymes to create non-ribosome peptides. Proteins are created by ribosomes through reactions that differ in detail from dehydration synthesis.
The five types of peptide bonds include Dipeptide, Tripeptide, Oligopeptide, Tetrapeptide, and Polypeptide.
To learn more about amino acids, refer to the link:
https://brainly.com/question/31442968
#SPJ4
Related Questions
the physical state of a reactant or product will affect the enthalpy of reaction in a thermochemical equation. t or f
Answers
The given statement "the physical state of a reactant or product will affect the enthalpy of reaction in a thermochemical equation," is false.
The heat of reaction is influenced by the physical state of the reactants and products.
What is thermochemical equation?The term "thermochemical equation" refers to a balanced chemical equation that not only specifies the quantities of the various reactants and products but also the quantity of heat generated or absorbed.
A thermochemical equation may be written using fractional coefficients.
H2 ( g ) + ½ O2 ( g ) ⇒H2O ( l ) +285.8 KJ mol-1
H2 ( g ) + ½ O2 ( g ) ⇒ H2O ( l ) ΔH = – 285.8 KJ mol-1
When 1 mole of hydrogen and 0.5 mole of oxygen react, 285.8 KJ mol-1 of heat is produced. The heat generated will also double if the reactant amounts are doubled.
2H2 ( g ) + O2 ( g ) → 2 H2O ( l ) + 571.6 KJ mol-1
2H2 ( g ) + O2 ( g ) → 2H2O ( l ) ,ΔH = – 571.6 KJ mol-1
What is the Heat of reaction (reaction enthalpy, or reaction enthalpy change)?The heat of reaction, enthalpy of reaction, or enthalpy change of reaction is the quantity of heat that is evolved or absorbed in a chemical reaction once the number of moles of reactants as represented by the chemical equation have completely reacted.
To learn more about thermochemical equation visit:
https://brainly.com/question/10384873
#SPJ1
Convert 4.50 e23 atoms of CO to grams.
Answers
Answer:
10.4664 grams of CO
Explanation:
Remark
There's a couple of things you must look out for in this question.
1. The use of the term atoms. There are 2 atoms in each mol of CO
2. You need to divide by 2 to find the number of molecules which will lead to moles.
Step one
Divide the number of atoms by 2
4.50 e^23 / 2 atoms = 2.25 * 10^23 molecules.
Step Two
Find the number of moles of CO
1 mol of anything is 6.02 * 10^23 molecules in this case
x = 2.25 * 10^23 molecules
1/x = 6.02 * 10^23/2.25 * 10 ^23 Cross multiply
1 * 2.25 * 10^23 = 6.02*10^23 * x Divide by 6.02 * 10^23
2.25 * 10 ^ 23 / 6.02 * 10^23 = x
x = .3738 moles of CO
Step Three
Find the gram molecular mass of CO
C = 12
O = 16
1 mole = 12 + 16 = 28 grams.
Step Four
Find the number of gram in 0.3738 mols
1 mol = 28 grams
0.3738 mol = x Cross multiply
x = 28 * 0.3738
x = 10.4664 grams
Which property would most likely be the same when comparing two random samples of copper?
A mass
O B. volume
OC weight
OD. density
Answers
Answer:
melting point and conductivity
Explanation:
as long as both samples are copper, it will melt at the same time, aswell as conduct electricity the same.
hi does anyone know what the answer to this is? i really need help

Answers
Answer:
The answer is
H. Met, Val, Lys, Arg, Gin, Ser
Explanation:
Alpha Centauri IS
star that is about the same size as the IS Sun. John is using tennis balls to model how the Sun and Alpha
Centauri appear from Earth. He places one tennis ball one meter to his right to model the Sun. He places another tennis ball ten
meters to his left to model Alpha Centauri.
What does John notice about the tennis ball that is ten meters to his left?
es
It looks wider than the other tennis ball.
BI
It looks smaller than the other tennis ball
It looks different in color than the other tennis ball
It looks different in shape than the other tennis ball

Answers
Answer:
it looks smaller for sure ^_^
(0,8) and (3,2) what the distance point ?
Answers
Answer:
2.4
Explanation:
3.2-0.8=2.4
is thi right i usefull
Which bond is most polar?
ОА СО
ов So
Осно
ODNO
Answers
Answer:
either first or second if not them try d but I'm pretty sure a also I'm sorry if I getbyou this wrong I dearly apologize
Select the equality for the following and 14 karat gold ring contains 58% gold by mass

Answers
Answer:
100g gold ring - 58g gold.
Explanation:
Assuming that the 100% represents the 100g of the gold ring, with a mathematical rule of three we can calculate the 58% of gold:
\(\begin{gathered} 100\%-100g \\ 58\%-x=\frac{58\%*100g}{100\%} \\ x=58g \end{gathered}\)So, in 100g of the gold ring, there are 58g of gold.
Fill in the table with the mass of each sample. Use the periodic table to find the molar mass of each compound. Then calculate moles and particles. Periodic TableThe two compounds have the same number of moles. Compare how the number of moles relates to the masses and number of particles for each compound.

Answers
1) First, let's calculate the molar mass of NaCl and CoCl₂:
Atomic mass of:
Na - 22.99 g/mol
Cl - 35.45 g/mol
Co - 58.93 g/mol
So for NaCl:
22.99 + 35.45 = 58.44 g/mol
Molar mass of NaCl = 58.44 g/mol
For CoCl₂:
58.93 + (2x35.45) = 129.83 g/mol
Molar mass of CoCl₂: 129.83 g/mol
2) Now let's find out the number of moles of each sample. For this, we use the following equation:
moles = mass / molar mass
NaCl:
mole = 116.8 / 58.44
mole = 1.999 moles of NaCl
CoCl₂:
mole = 259.7 / 129.83
mole = 2.000 moles of CoCl₂
3) To find the amount of particles, we use the Avogadro's constant. Avogadro's constant is a constant of proportion between the amount of matter and the number of entities that are linked to that amount. These entities can be atoms, molecules, ions, electrons, protons, neutrons.
In this case, we have the same number of moles, so we will have the same number of particles for both NaCl and CoCl₂.
explain, in terms of lechatliers principal why the final concetration of nh3 is greater than the inital concentration of nh3
Answers
The final concentration of NH₃ is greater than the initial concentration due to Le Chatelier's principle, which states that when a system at equilibrium is subjected to a change in concentration, pressure, or temperature, the system will adjust to counteract the change and restore equilibrium.
In the context of the Haber process, where nitrogen (N₂) and hydrogen (H₂) react to form ammonia (NH₃), the reaction can be represented as:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
When the initial concentration of NH₃ is increased, according to Le Chatelier's principle, the system will try to counteract the change by shifting the equilibrium position to reduce the concentration of NH₃. This can be achieved by favoring the reverse reaction, in which NH₃ is consumed, and N₂ and H₂ are produced.
On the other hand, if the initial concentration of NH₃ is decreased, the system will attempt to increase the concentration of NH₃ to restore equilibrium. It does this by shifting the equilibrium position in the direction of the forward reaction, in which N₂ and H₂ react to form NH₃. This results in a higher final concentration of NH₃ than the initial concentration, as the system adjusts to counteract the change and restore equilibrium.
Learn more about equilibrium here:
https://brainly.com/question/30807709
#SPJ11
the influence of a cerium additive on ultrafine diesel particle emissions and kinetics of oxidation.
Answers
The influence of a cerium additive on ultrafine diesel particle emissions and kinetics of oxidation can be significant. Cerium additives, such as cerium oxide (CeO2), are often used in diesel fuel as combustion catalysts to improve fuel efficiency and reduce emissions.
When cerium additives are introduced into diesel fuel, they can enhance the oxidation process of diesel particles. The cerium particles act as catalysts, promoting the conversion of hydrocarbons and carbon monoxide into less harmful gases like carbon dioxide. This helps to reduce the emissions of ultrafine particles, which are known to have adverse health effects.
The addition of cerium can also influence the kinetics of oxidation. It can lower the activation energy required for the oxidation reaction to occur, thereby increasing the rate of oxidation. This means that the diesel particles can be oxidized more quickly, leading to a reduction in particle emissions.
Furthermore, cerium additives have been found to have regenerative properties. They can form a protective layer on the surface of diesel particles, preventing the aggregation and growth of particles. This can help to maintain the small size of ultrafine particles, which are less harmful to human health compared to larger particles.
In conclusion, the addition of a cerium additive to diesel fuel can have a positive impact on ultrafine diesel particle emissions and the kinetics of oxidation. It can enhance the oxidation process, reduce particle emissions, and prevent particle growth. However, it is important to note that the specific effects may vary depending on the concentration of the additive, engine conditions, and other factors.
Learn more about oxidation here:-
https://brainly.com/question/16976470
#SPJ11
g 1 mole of a monoatomic gas increases its volume from 1 to 3 while the pressure also increases from 250 to 1000. this p-v process is linear. find the involved work of this process
Answers
The involved work done when gas increases its volume from 1 to 3 while the pressure also increases from 250 to 1000 is 2250 joule
Work done= ΔpΔv
Work done=3×750
Work done=2250 joule
First Law of Thermodynamics: The change in total internal energy of a particular system between two states is described by the first law of thermodynamics. The first law of thermodynamics states that when heat is added to a system or when work is done, the total internal energy of the system will change. A positive result when calculating work done indicates that energy is discharged into the environment while the system is operating. If the value is negative, the environment's influence has caused the system to gain energy.
To know more about work done visit : brainly.com/question/10678395
#SPJ4
90 Strontium 38 Sr has a half-life of 29.1 yr. It is chemically similar to calcium, enters the body through the food chain, and collects in the bones. Consequently, 30 Sr is a particularly serious health hazard. How long (in years) will it take for 99.9049% of the Sr released in a nuclear reactor accident to disappear? 38 Number i Units
Answers
The time it will take for 99.9049% of the released Sr-90 to disappear is approximately 96.93 years.
To calculate this, we can use the concept of half-life. The half-life of Sr-90 is given as 29.1 years. The percentage of Sr-90 that remains after a certain number of half-lives can be calculated using the formula:
Remaining percentage = (1/2)^(number of half-lives)
To determine the time it will take for 99.9049% of the Sr-90 to disappear, we can use the concept of half-life.
Given:
Half-life of Sr-90 (t₁/₂) = 29.1 years
Remaining percentage (R) = 0.099049 (99.9049%)
We can use the formula:
time = (number of half-lives) * (half-life of Sr-90)
To calculate the number of half-lives, we can use the equation:
R = (1/2)^(number of half-lives)
Taking the logarithm of both sides:
log(R) = (number of half-lives) * log(1/2)
Substituting the values:
log(0.099049) = (number of half-lives) * log(1/2)
Solving for the number of half-lives:
(number of half-lives) = log(0.099049) / log(1/2)
Now we can calculate the time:
time = (number of half-lives) * (half-life of Sr-90)
Substituting the given values:
time = (log(0.099049) / log(1/2)) * 29.1
To simplify the expression, let's evaluate the logarithms and perform the calculations:
log(0.099049) ≈ -1.003
log(1/2) ≈ -0.301
Using these values, we can simplify the expression:
time ≈ (-1.003 / -0.301) * 29.1
Simplifying further:
time ≈ 3.33 * 29.1
Calculating the product:
time ≈ 96.93
Therefore, it will take approximately 96.93 years for 99.9049% of the Sr released in a nuclear reactor accident to disappear.
You can learn more about Sr-90 at
https://brainly.com/question/1581557
#SPJ11
While resting, the average 70-kg human male consumes 14 L of pure O2 per hour at 25 °C and 100 kPa. How many moles of O2 are consumed by a 70 kg man while resting for 1.0 h?
Answers
The average 70-kg human male consumes 0.57 moles.
We will use ideal gas equation to find the number of moles. It is as follows.
PV = nRT, where P is pressure, V is volume, R is gas constant, T is temperature and n is number of moles.
R = 8.3 Pa m³/K/mol
P = 100 kPa which will be 10⁵ Pa
V = 14 litre or 0.014 m³
T = 273 + 25
T = 298 K
Keep the values in formula to find number of moles
n = (10⁵ × 0.014)/(8.3 × 298)
Performing multiplication in numerator and denominator on Right Hand Side of the equation
n = 1400/2473.4
Performing division on Right Hand Side of the equation
n = 0.57 moles
Thus, 0.57 moles are being consumed.
Learn more about moles -
https://brainly.com/question/15356425
#SPJ4
what is a dependent variable?
Answers
Important marine autotrophs that have silica incorporated into their cell walls are: a. coccolithophorids. b. dinoflagellates.c. radiolarians.d. diatoms.
Answers
The autotrophs that have silica incorporated into their cell walls are diatoms. Option(D)
Diatoms are a type of microalgae found in both freshwater and marine environments. They have a unique external skeleton called a frustule, which is composed of silica.
The frustule provides structural support and protection to the diatom cell. Coccolithophorids, dinoflagellates, and radiolarians are other types of marine microorganisms but do not have silica in their cell walls.
Autotrophs are organisms capable of producing their own food using energy from sunlight (photosynthesis) or inorganic chemicals (chemosynthesis). They convert raw materials into organic compounds, serving as the primary producers in ecosystems.
Coccolithophorids are a group of single-celled algae found in marine environments. They are characterized by tiny calcified plates called coccoliths that cover their cell surface. These coccoliths play a role in carbon cycling and are significant contributors to marine sediment and biogeochemical processes.
To learn more about autotrophs refer here:
https://brainly.com/question/15192191#
#SPJ11
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many moles of water can be made when 146.3 grams of HNO3 are consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.:
Element
Molar Mass
Hydrogen
1
Nitrogen
14
Copper
63.5
Oxygen
16
Answers
The given chemical equation is: 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O1Oxygen16Now, let us balance the given chemical equation by following the Law of Conservation of Mass.Balancing the chemical equation: 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O1Oxygen16Number of atoms on both sides of the chemical equation should be the same. Let's check one by one.Number of Copper (Cu) atoms:
On the left side: 3 atomsOn the right side: 3 atomsNumber of Nitrogen (N) atoms:On the left side: 24 atoms (8 × 3)On the right side: 6 atoms (2 × 3)Number of Oxygen (O) atoms:On the left side: 24 atoms (8 × 3)On the right side: 24 atoms (2 × 3 + 4 × 4 + 16)All the elements on both sides are equal, so the given chemical equation is balanced.The chemical reaction is a redox reaction in which Copper acts as a reducing agent while Nitric Acid acts as an oxidizing agent. Redox reaction is a type of chemical reaction in which both oxidation and reduction processes occur simultaneously. In the given chemical equation, Copper (Cu) gets oxidized to Copper Nitrate [Cu(NO3)2] by Nitric Acid (HNO3) by donating electrons. It loses two electrons to form Cu(NO3)2.Cu → Cu2+ + 2e- (oxidation)On the other hand, Nitric Acid (HNO3) gets reduced to Nitrogen Monoxide (NO) by accepting electrons. It gains two electrons to form NO.4H+ + 2NO3- + 8e- → 2NO + 4H2O (reduction)Thus, Copper (Cu) acts as a reducing agent while Nitric Acid (HNO3) acts as an oxidizing agent in the given redox reaction.For such more question on chemical equation
https://brainly.com/question/26694427
#SPJ8
Roberto wants to measure the lengths of the sides
of a metal cube. Which tool should he use?
Answers
Answer:
ruler
Explanation:
1. H2SO4, sulfuric acid, contains three different types of atoms: hydrogen (H), sulfur (S), and oxygen (O). Each of these atoms represents a different . Since the three types are combined in a fixed ratio, this means that H2SO4 is a(n) molecule. 2. The smallest unit of matter that retain all of the physical properties of that type of matter is a(n) atom. 3. is anything that occupies space and/or has any substance. 4. If two or more atoms are bonded together, they form a(n) . 5. The scientific study of matter is called . 6. Within a plant, water (H2O) and carbon dioxide (CO2) can be combined (using the energy of sunlight) to produce glucose (C6H12O6) and oxygen (O2). If you were to write out this chemical reaction, water and carbon dioxide are each an example of a(n) while glucose and
Answers
Answer:
The blanks can be completed with words in the following sequence
1) elements
compound
2) atom
3) matter
4) compound
5) chemistry
6) reactants
product
Explanation:
In H2SO4, atoms of hydrogen, sulphur and oxygen represent different elements that are combined to form the compound.
The smallest indivisible unit of matter that still retains all the properties of matter is an atom. Matter is anything that has weight and occupy space.
Atoms combine in fixed ratios to form compounds. Chemistry studies matter scientifically and pays specific attention to the changes that matter undergoes.
Considering the reaction of photosynthesis, water and carbon dioxide combine to give glucose so the water and carbon dioxide are reactants while the glucose is the product of the reaction.
if an object has density of 0.75 g/milliliters then which is greater the mass or the volume?
Answers
Answer:
Volume
Explanation:
The formula for density is m/v so if the mass was greater, it would be more than one. It is less than one so the volume is greater.
How many mmol of iron are there in 650 mg of iron? O A. 11.6 mmol Fe B. 363.02 mmol Fe C. 55.85 mmol Fe D. 8.95 mmol Fe
Answers
There are 11.6 mmol of iron in 650 mg of iron.
Given the mass of iron as 650 mg. The molar mass of iron is 55.85 g/mol.
We need to calculate how many millimoles (mmol) are present in the given amount of iron.
We will use the following conversion:
1 g = 1000 mg
1 mol = molar mass in grams
1 mmol = 0.001 mol
Number of moles of iron
= 650 mg ÷ 1000 mg/g
= 0.65 g ÷ 55.85 g/mol
= 0.0116 mol
Number of millimoles of iron
= 0.0116 mol ÷ 0.001 mol/mmolar mass of iron
= 11.6 mmol
Hence, there are 11.6 mmol of iron in 650 mg of iron. Therefore, the correct option is A. 11.6 mmol Fe.
Learn more about the millimoles from the given link-
https://brainly.com/question/30640148
#SPJ11
Complex organisms require a large number of cells that
A.Complex organisms require a large number of cells that
Answers
That’s the answer to your question
I WILL GIVE YOU BRAINLIEST HELP HELP HELP THE SUBJECT IS ACTUALLY SCIENCE What factor is deer in an ecosystem? Abiotic or Biotic? the subject is actually science
Answers
Answer: the answer is biotic because dear are a living factor
Explanation:
Fill in the blanks
The _____________ _____________ is the atomic mass rounded to a whole number.
Answers
The mass number is the atomic mass rounded to a whole number, i.e., which is a value that rounds the atomic weight to a near number.
What is mass number?The expression mass number is used in chemistry to denote the total amount of subatomic particles i.e. atomic protons and neutrons, which are present in a given atom (for example hydrogen has only one proton and one neutron).
Therefore, with this data, we can see that mass number denotes the overall amount of protons and neutrons present in a given atom, which is equal to one in the hydrogen atom
Learn more about the mass number here:
https://brainly.com/question/28409714
#SPJ1
Write the molecular formulas of alkane,alkene with one double bond, alkene with two double bonds,Cycloalkane and Cycloalkenenwith no substituents comprising six carbons.

Answers
There are two types of chemical compound one is covalent compound and other is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. Therefore, chemical formula can be shown as below.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
An ionic compound is a metal and nonmetal combined compound. Ionic compound are very hard. They have high melting and boiling point because of strong ion bond.
Compound chemical formula
alkane CnH2n+2
alkene with one double bond CH\(_2\)=CH\(_2\)
alkene with two double bond CH\(_2\)=c=CH\(_2\)
cycloalkane C\(_6\)H\(_{12}\)
Therefore, chemical formula can be shown as above.
To learn more about chemical compound, here:
brainly.com/question/26487468
#SPJ1
decide which element probably forms a compound with hydrogen that has a chemical formula most and least similar to the chemical formula of the compound formed by hydrogen and selenium.potassium,sulfur,chlorine,fluorine
Answers
The element that probably forms a compound with hydrogen that has a chemical formula most and least similar to the chemical formula of the compound formed by hydrogen and selenium is sulfur.
Sulfur is in the same group as selenium, which means they have similar chemical properties. Therefore, sulfur would most likely form a compound with hydrogen that has a chemical formula similar to hydrogen selenide (H₂Se), the compound formed by hydrogen and selenium. On the other hand, chlorine and fluorine are both highly reactive nonmetals that would not likely form compounds with hydrogen in a similar way to selenium.
Potassium is a highly reactive metal and would not likely form a compound with hydrogen in a similar way to selenium either.
To know more about element visit :-
https://brainly.com/question/20096027
#SPJ11
i need help pls!!!!!!!!!!!
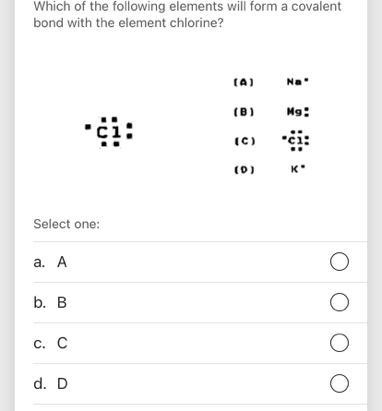
Answers
Which element will form covalent bonds with chlorine?
A. Carbon. YES. C is a nonmetal. B. Magnesium. NO. Mg is a metal. C. Potassium. NO. K is a metal. D. Aluminum. NO. Al is a metal.
Chlorine will form covalent bonds with A. Carbon .

Molecular scale for gas
Answers
as a system reaches thermal equilibrium, _____ has been transferred from areas of high to low _____.
Answers
As a system reaches thermal equilibrium, heat has been transferred from areas of high to low temperature.
In other words, when a system reaches thermal equilibrium, there is no longer a temperature difference between its different parts, and the heat has been evenly distributed throughout the system. This can happen through a variety of mechanisms, such as conduction, convection, and radiation, and the specific mechanism will depend on the properties of the system and the materials it is made of. Once thermal equilibrium has been reached, the system will remain at a constant temperature until something changes, such as the addition or removal of heat or a change in the materials or structure of the system.
to know more about heat-
https://brainly.com/question/13860901
#SPJ4
What do all of the inventors discussed in this lesson have in common?
1. All of the inventors can be characterized as problem-solvers.
2. All of the inventors used engineering principles to create new things.
3. All of the inventors created new things as children.
4. All of the inventors needed mathematics principles to formulate solutions.
PLEASE I NEED helppp