Answers
Answer:
Definition. Gram molecular mass is the mass in grams of one mole of a molecular substance. Gram molecular mass is the same as molar mass. The only difference is that gram molecular mass specifies the mass unit to be used. Gram molecular mass may be reported in grams or grams per mole (g/mol).
Related Questions
What would the approximate age of an
igneous rock that contains only 1/4 of its
original carbon-14 (half-life of carbon is
5700 years)
Answers
Explanation:
Carbon-14 has a half life of 5730 years, meaning that 5730 years after an organism dies, half of its carbon-14 atoms have decayed to nitrogen atoms. Similarly, 11460 years after an organism dies, only one quarter of its original carbon-14 atoms are still around.
Discuss a specific subculture to which you belong. What is it and why is it a subculture? Describe the social structures of the subculture. Is it analagous to, or different from, the dominant social structure of American society? Be sure to substantiate your dicussion with facts and examples.
Answers
There are numerous subcultures, such as hippies, anti-gun groups, high school jocks, environmental activists, people in the furry community, people in the cosplay community, punks, goths, and many more.
What are subcultures ?"An identifiable subgroup within a society or group of people, particularly one distinguished by beliefs or interests that differ from those of the larger group."
Subcultures are important in articulating an identity, creating a sense of belonging, and influencing members to think about their relationship to mainstream society; however, subcultures differ from widely recognized identity categories such as ethnicity.
Subcultures exist within a society's dominant culture. Subcultures in America include hippies, punk rockers, beatniks, and hipsters.
Thus, There are numerous subcultures, such as hippies, anti-gun groups, high school jocks, environmental activists, people in the furry community.
To learn more about subculture, follow the link;
https://brainly.com/question/7243272
#SPJ1
. 2.514g Ca, 1.004g O empirical formula
Answers
The empirical formula of the given compound is \(CaO\).
A chemical compound's empirical formula in chemistry is the simplest whole number ratio of atoms within a molecule.
Given:
Mass of Ca = 2.514g
Mass of O = 1.004g
Molar mass of Ca = 40.078g/mol
Molar mass of O = 15.999g/mol
To find:
Empirical formula = ?
Formula:
Empirical formula = Moles of Ca : Moles of O
Calculations:
No. of moles of Ca = 2.514 / 40.078
n of Ca = 0.06273 moles
No. of moles of O = 1.004 / 15.999
n of O = 0.06275 moles
Divide both by the lowest no. of moles
n of Ca : n of O = 1 : 1
Empirical formula = \(CaO\)
Result:
\(CaO\) is the empirical formula of the compound.
The complete question is:
In an experiment, a 2.514 g sample of calcium was heated in a pure stream of oxygen, and was found to increase in mass by 1.004 g. Calculate the empirical formula for the given compound.
Learn more about empirical formula here:
https://brainly.com/question/14044066
#SPJ9
Consider the chemical equation.
2H2 + O2 Right arrow. 2H2O
What is the percent yield of H2O if 87.0 g of H2O is produced by combining 95.0 g of O2 and 11.0 g of H2?
Use Percent yield equals StartFraction actual yield over theoretical yield EndFraction times 100..
Answers
2H2 + O2 Right arrow. 2H2O
What is the percent yield of H2O if 87.0 g of H2O is produced by combining 95.0 g of O2 and 11.0 g of H2?
Use Percent yield equals StartFraction actual yield over theoretical yield EndFraction times 100..
In an investigation of the decomposition of water, a student compared the amount
of oxygen in one test tube to the amount of hydrogen in the other test tube. Which
of the following statements is correct about the comparison?
A. There is twice as much oxygen as hydrogen.
B. There is an equal amount of oxygen and hydrogen.
C. There is one-half as much oxygen as hydrogen.
D. There is four times as much oxygen as hydrogen.
Answers
In an investigation of the decomposition of water, a student compared the amount of oxygen in one test tube to the amount of hydrogen in the other test tube is There is one - half as much as hydrogen.
In decomposition of water , water can be broken into the hydrogen gas and the oxygen gas. The water decomposition reaction is given as :
2H₂O ------> 2H₂ + O₂
The ratio of hydrogen and oxygen in the decomposition reaction of water is 2 : 1.
Thus, In an investigation of the decomposition of water, a student compared the amount of oxygen in one test tube to the amount of hydrogen in the other test tube is There is one - half as much as hydrogen.
To learn more about decomposition here
https://brainly.com/question/8009068
#SPJ1
Every neutral atom of a given element has the same number of what two subatomic particles?
A. Protons and neutrons
B. Protons and electrons
Answers
Answer:
protons and electrons
Explanation:
because two atoms of the same elements have different(mass)isotope so the answer is B
How many atoms of oxygen are in one molecule of water (H2O)? one two four three
Answers
Answer:
there is one atom of oxygen and two atoms of hydrogen
Explanation:
where is a tornado most likey from

Answers
Answer:
If I'm correct i believe that the answer is A.
Answer:
A. Where warm moist air masses traveling north collide with cool dry air masses traveling south
Explanation:
I got it right
The rate constant, k, for a reaction is 0.0354 sec1 at 40°C. Calculate the rate constant for the
same reaction at 125°C if the activation energy is 26.5 kJ/mol.
Answers
Answer:
The rate constant of the reaction at 125˚ is \(0.3115 \ \text{sec}^{-1}\).
Explanation:
The Arrhenius equation is a simple equation that describes the dependent relationship between temperature and the rate constant of a chemical reaction. The Arrhenius equation is written mathematically as
\(k \ = \ Ae^{\displaystyle\frac{-E_{a}}{RT}}\)
\(\ln k \ = \ \ln A \ - \ \displaystyle\frac{E_{a}}{RT}\)
where \(k\) is the rate constant, \(E_{a}\) represents the activation energy of the chemical reaction, \(R\) is the gas constant, \(T\) is the temperature, and \(A\) is the frequency factor.
The frequency factor, \(A\), is a constant that is derived experimentally and numerically that describes the frequency of molecular collisions and their orientation which varies slightly with temperature but this can be assumed to be constant across a small range of temperatures.
Consider that the rate constant be \(k_{1}\) at an initial temperature \(T_{1}\) and the rate constant \(k_{2}\) at a final temperature \(T_{2}\), thus
\(\ln k_{2} \ - \ \ln k_{1} = \ \ln A \ - \ \displaystyle\frac{E_{a}}{RT_{2}} \ - \ \left(\ln A \ - \ \displaystyle\frac{E_{a}}{RT_{1}}\right) \\ \\ \\ \rule{0.62cm}{0cm} \ln \left(\displaystyle\frac{k_{2}}{k_{1}}\right) \ = \ \displaystyle\frac{E_{a}}{R}\left(\displaystyle\frac{1}{T_{1}} \ - \ \displaystyle\frac{1}{T_{2}} \right)\)
\(\rule{1.62cm}{0cm} \displaystyle\frac{k_{2}}{k_{1}} \ = \ e^{\displaystyle\frac{E_{a}}{R}\left(\displaystyle\frac{1}{T_{1}} \ - \ \displaystyle\frac{1}{T_{2}} \right)} \\ \\ \\ \rule{1.62cm}{0cm} k_{2} \ = \ k_{1}e^{\displaystyle\frac{E_{a}}{R}\left(\displaystyle\frac{1}{T_{1}} \ - \ \displaystyle\frac{1}{T_{2}} \right)}\)
Given that \(E_{a} \ = \ 26.5 \ \ \text{kJ/mol}\), \(R \ = \ 8.3145 \ \ \text{J mol}^{-1} \ \text{K}^{-1}\), \(T_{1} \ = \ \left(40 \ + \ 273\right) \ K\), \(T_{2} \ = \ \left(125 \ + \ 273\right) \ K\), and \(k_{1} \ = \ 0.0354 \ \ \text{sec}^{-1}\), therefore,
\(k_{2} \ = \ \left(0.0354 \ \ \text{sec}^{-1}\right)e^{\displaystyle\frac{26500 \ \text{J mol}^{-1}}{8.3145 \ \text{J mol}^{-1} \ \text{K}^{-1}}\left(\displaystyle\frac{1}{313 \ \text{K}} \ - \ \displaystyle\frac{1}{398 \ \text{K}} \right)} \\ \\ \\ k_{2} \ = \ 0.3115 \ \ \text{sec}^{-1}\)
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
what is the complex equation for copper sulfate and sodium hydroxide reaction?
Answers
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4
There you go, happy now? But I have a feeling that you don't actually understand what this equation means or how the reaction works. So, before you start pretending to be a chemist again, maybe actually learn some basic chemistry first.
Cuso4 + NaoH -》cu(oH)2 +Na2So4
Cuso4 + 2NaoH -》cu(oH)2 +Na2So4
Explanation:
this is balanced equation
Q)Indicate True and False statements:
a. The melting points of saturated fatty acids increase with increasing chain length
b. Double bonds in saturated fatty acids are separated by -CH2-CH2-groups
c. △9, 12-all cis, 18:3 is linoleic acid
d. A by-product of the hydrolysis of fats is glycerol
Answers
Statement a is true, as the melting points of saturated fatty acids do increase with increasing chain length. Statement b is false, as saturated fatty acids do not contain double bonds. Statement c is false, as △9, 12-all cis, 18:3 represents alpha-linolenic acid, not linoleic acid. Statement d is true, as glycerol is indeed a by-product of the hydrolysis of fats.
a. True. The melting points of saturated fatty acids increase with increasing chain length. This is because longer fatty acid chains have stronger intermolecular forces, such as van der Waals forces, which require more energy to break and result in higher melting points.
b. False. Saturated fatty acids do not have double bonds. They are composed of only single carbon-carbon bonds. Double bonds are found in unsaturated fatty acids.
c. False. △9, 12-all cis, 18:3 is not linoleic acid. It represents the structure of alpha-linolenic acid. Linoleic acid is △9, 12-18:2, which means it has two double bonds located at the 9th and 12th carbon positions.
d. True. A by-product of the hydrolysis of fats is glycerol. When fats undergo hydrolysis, they are broken down into their constituent fatty acids and glycerol. Glycerol is a three-carbon molecule that is a component of triglycerides (fats).
During hydrolysis, the ester bonds between the fatty acids and glycerol are cleaved, resulting in the release of fatty acids and glycerol.
For more such question on double bonds visit:
https://brainly.com/question/27879143
#SPJ8
A chemist titrates 0.200 M NaOH, strong base, with 50.00 ML of 0.100 M HCI, strong acid. How many mL of NaOH will be required to titrate to the endpoint
Answers
Answer:
25 mL of NaOH
Explanation:
We'll begin by writing the balanced equation for the reaction. This is illustrated below:
HCl + NaOH —> NaCl + H₂O
From the balanced equation above,
Mole ratio of the acid, HCl (nₐ) = 1
Mole ratio of the base, NaOH (n₆) = 1
Finally, we shall determine the volume of NaOH required for the reaction. This can be obtained as follow:
Molarity of the base, NaOH (M₆) = 0.2 M
Molarity of the acid, HCl (Mₐ) = 0.1 M
Volume of the acid, HCl (Vₐ) = 50 mL
Mole ratio of the acid, HCl (nₐ) = 1
Mole ratio of the base, NaOH (n₆) = 1
Volume of the base, NaOH (V₆) =?
MₐVₐ / M₆V₆ = nₐ / n₆
0.1 × 50 / 0.2 × V₆ = 1
5 / 0.2 × V₆ = 1
Cross multiply
5 = 0.2 × V₆
Divide both side by 0.2
V₆ = 5/0.2
V₆ = 25 mL
Thus, 25 mL of NaOH is needed for the reaction
how the El Niño event affected the weather, food production, water supply, or human health?
El Niño was responsible for the following events in 2015:
16 tropical cyclones in the central Pacific hurricane basin
three category 4 hurricanes occurred at the same time
emergency water rationed in St. Lucia and San Juan
65 percent of Antigua's farmers went out of business
northern, central, and southeastern Ethiopian highlands received 50–90 percent of their normal rainfal.
Answers
Answer:
EL Niño had hurricanes and so many damages in nature. :)
Explanation:
it creates warm weather, there is low pressure towards canada
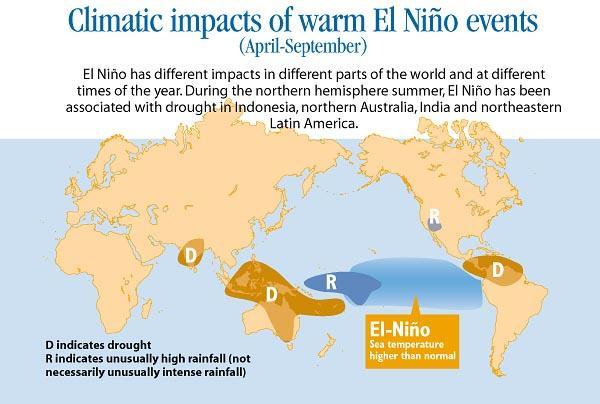
Numerous health issues, such as disease outbreaks, malnutrition, heat stress, and respiratory illnesses are being brought on by the severe drought and associated food insecurity, flooding, precipitation, and temperature increases brought on by El Nino.
What is El- Nino?The exceptional warming of surface waters in the eastern tropical Pacific Ocean is referred to as El Nino, a climate pattern. El Nino is a bigger phenomena known as the El Nino-Southern oscillation, and it is its warm phase.
Climate change brought on by El Nino can be severe and extensive. Increased precipitation results from convection over warmer surface waters. In Ecuador and northern Peru, rainfall rises dramatically, causing coastal erosion and flooding.
Homes, businesses, schools, and hospitals may be destroyed by heavy rains and flooding. They hinder transportation and obliterate crops. As reservoirs dry up and rivers carry less water, the droughts pose a threat to the area's water resources. Agriculture is in danger since it depends on water for irrigation.
Find more on El-Nino:
https://brainly.com/question/18307318
#SPJ2
Suppose a student starts with 2.4319 g of a sand mixture and separates the components into 1.3012 g of NaClNaCl , 0.5410 g of SiO2 , and 0.4503 g of CaCO3 . Based on the amount of recovered, what is the percent of SiO2 in the starting mixture?
Answers
The starting mixture contained approximately 23.62% SiO2.
What is the use of SiO2?Silicon dioxide (SiO2) has many important uses in various fields:
Glassmaking: SiO2 is a primary component of most types of glass. It is added to glass to improve its hardness, clarity, and resistance to heat and chemicals.
Ceramics: SiO2 is used in the production of ceramics and pottery as it gives the material added strength and durability.
Electronics: SiO2 is used as a dielectric material in electronic devices like transistors, integrated circuits, and microchips. It is an important component of the insulation layers that protect the electrical components and prevent them from overheating.
Construction: SiO2 is used as an important component in construction materials like concrete, bricks, and roofing tiles. Its hardness and durability make it ideal for building materials.
Cosmetics: SiO2 is used in many cosmetic products like face powders, sunscreens, and lotions. It is used as an absorbent or bulking agent that helps to give products a silky texture.
To determine the percentage of SiO2 in the starting mixture, we need to calculate the total mass of the starting mixture and the mass of SiO2 in it.
The total mass of the starting mixture is the sum of the masses of NaCl, SiO2, and CaCO3:
total mass = 1.3012 g + 0.5410 g + 0.4503 g = 2.2925 g
The mass of SiO2 in the starting mixture is given as 0.5410 g.
To calculate the percentage of SiO2 in the starting mixture, we divide the mass of SiO2 by the total mass of the mixture and multiply by 100:
% SiO2 = (mass of SiO2 / total mass) x 100
% SiO2 = (0.5410 g / 2.2925 g) x 100
% SiO2 = 23.62%
To know more about Silicon dioxide, visit:
https://brainly.com/question/15412188
#SPJ1
Given the partial equation: MnO4−+ SO32− → Mn2++ SO42−, balance the reaction in acidic solution using the half-reaction method and fill in the coefficients. The missing blanks represent H2O, H+, or OH-, as required to balance the reaction. Enter the coefficients as integers, using the lowest whole numbers. If the coefficient for something is "1", make sure to type that in and not leave it blank. Enter only the coefficients.
Answers
Explanation:
MnO4−+ SO32− → Mn2++ SO42−
Splitting into half equations;
MnO4− → Mn2+
SO32− → SO42−
Balancing the electrons;
2 MnO4− + 10 e- → 2Mn2+
5SO32− → 5SO42− + 10 e-
In an acidic medium, it becomes;
2 MnO4− + 8 H+ → 2 Mn2+ + 4 H2O
5 SO32− + H2O → 5 SO42− + 2 H+
Final equation is;
2 MnO4- + 5 SO32- + 6 H+ → 2 (Mn)2+ + 5 SO42- + 3 H2O
Coefficient of H+ = 6
Coefficient of H2O = 3
Coefficient of MnO4- = 2
Coefficient of SO32- = 5
Coefficient of (Mn)2+- = 2
Coefficient of SO42- = 5
Answer:
\(5SO_3^{2-}+2MnO_4^{-}+6H^+ \rightarrow 5SO_4^{2-}+ 2Mn^{2+}+3H_2O\)
Explanation:
Hello,
In this case, given the reaction:
\(MnO_4^{-}+ SO_3^{2-} \rightarrow Mn^{2+}+ SO_4^{2-}\)
We first identify the oxidation state of both manganese and sulfur at each side:
\(Mn^{7+}O_4^{-}+ S^{4+}O_3^{2-} \rightarrow Mn^{2+}+ S^{6+}O_4^{2-}\)
So we have the oxidation and reduction half-reactions below, including the addition of water and hydronium as it is in acidic media:
\(S^{4+}O_3^{2-}+H_2O \rightarrow S^{6+}O_4^{2-}+2H^++2e^-\)
\(Mn^{7+}O_4^{-}+8H^++5e^- \rightarrow Mn^{2+}+4H_2O\)
Next, we exchange the transferred electrons:
\(5*(S^{4+}O_3^{2-}+H_2O \rightarrow S^{6+}O_4^{2-}+2H^++2e^-)\\2*(Mn^{7+}O_4^{-}+8H^++5e^- \rightarrow Mn^{2+}+4H_2O)\\\\5S^{4+}O_3^{2-}+5H_2O \rightarrow 5S^{6+}O_4^{2-}+10H^++10e^-\\2Mn^{7+}O_4^{-}+16H^++10e^- \rightarrow 2Mn^{2+}+8H_2O\)
Then we add the resulting half-reactions and simplify the transferred electrons:
\(5S^{4+}O_3^{2-}+5H_2O+2Mn^{7+}O_4^{-}+16H^+ \rightarrow 5S^{6+}O_4^{2-}+10H^++ 2Mn^{2+}+8H_2O\)
We rearrange the terms in order to simplify water and hydronium molecules:
\(5S^{4+}O_3^{2-}+2Mn^{7+}O_4^{-}+16H^+-10H^+ \rightarrow 5S^{6+}O_4^{2-}+ 2Mn^{2+}+8H_2O-5H_2O\\\\5S^{4+}O_3^{2-}+2Mn^{7+}O_4^{-}+6H^+ \rightarrow 5S^{6+}O_4^{2-}+ 2Mn^{2+}+3H_2O\)
Finally we write the balanced reaction in acidic media:
\(5SO_3^{2-}+2MnO_4^{-}+6H^+ \rightarrow 5SO_4^{2-}+ 2Mn^{2+}+3H_2O\)
Best regards.
For the following problem convert both the reactants to moles and balance chemical equationsThe reaction of 167 g Fe2O3 with 85.8 g CO produces. 72.3g Fe. START to determine the limiting reactant Fe2O3+CO—->Fe+CO2
Answers
Let's start by balancing the reaction:
\(Fe_2O_3+CO\longrightarrow Fe+CO_2\)As we can see, C appears only on two comopunds, CO and CO₂, and since both have 1 C each, their coefficients have to be the same for C to be balanced. However, CO has 1 O and CO₂ has 2, so there is a difference of 1 O betwee them.
The other source of O is Fe₂O₃, that has 3 O. So, we must choose a coefficient for CO and CO₂ such that the difference between the numbers of O is a multiple of 3, that way we can fix this difference with the O from Fe₂O₃. So, we can put coefficients of 3 on both of them:
\(Fe_2O_3+3CO\longrightarrow Fe+3CO_2\)That way, we maintained C balanced (3 on each side) and now we have 3 + 3 O on the left side and 6 O on the right side, so the same amount.
Now, we just have to calance Fe, but it is easy since we have it alone in Fe. Since we have 2 on the left side, it is enough to put a coefficient of 2 on Fe to get the balanced reaction:
\(Fe_2O_3+3CO\longrightarrow2Fe+3CO_2\)Now, to convert from mass to number of moles, we need the molar masses of the reactants, which we can calculate from the atomic weights of the elemnts in each of them:
\(M_{Fe_2O_3}=2\cdot M_{Fe}+3\cdot M_O=(2\cdot55.845+3\cdot15.9994)g/mol=159.6882g/mol\)\(M_{CO}=1\cdot M_C+1\cdot M_O=(1\cdot12.0107+1\cdot15.9994)g/mol=28.0101g/mol\)Now, we can convert their masses to number of moles:
\(\begin{gathered} M_{Fe_{2}O_{3}}=\frac{m_{Fe_2O_3}}{n_{Fe_{2}O_{3}}} \\ n_{Fe_2O_3}=\frac{m_{Fe_2O_3}}{M_{Fe_{2}O_{3}}}=\frac{167g}{159.6882g/mol}=1.045787\ldots mol \end{gathered}\)\(\begin{gathered} M_{CO}=\frac{m_{CO}}{n_{CO}} \\ n_{CO}=\frac{m_{CO}}{M_{CO}}=\frac{85.8g}{28.0101g/mol}=3.063180\ldots mol \end{gathered}\)Now, to determine the limiting reactant, we need to divide both the number of mole by their coefficients on the balanced reaction, so we can see how many we need per reaction of each:
\(\begin{gathered} Fe_2O_3\colon\frac{n_{Fe_2O_3}}{1}=\frac{1.045787\ldots mol}{1}=1.045787\ldots mol \\ CO\colon\frac{n_{CO}}{3}=\frac{3.063180\ldots mol}{3}=1.021060\ldots mol \end{gathered}\)Now, the limiting reactant is the one we have less number of moles per reaction. We can see that we have less CO than Fe₂O₃, so the limiting reactant is CO.
Order the structures from smallest to largest. Chloroplast Leaf Glucose molecule Cell Water molecule
Answers
Answer:
water molecule...glucose..chloroplast...cell...leaf
The structure of the order from smallest to the largest should be shown below:
The order of the series are as follows:
Water moleculeGlucoseChoroplastCellLeafLearn more: https://brainly.com/question/26115859?referrer=searchResults
is a one time explanation of one observation a hypothesis and a scientific theory 100 points
Answers
One time explanation of one observation is a hypothesis.
Whenever, an assumption is made juts by observation and without proper testing then that is referred as an hypothesis.
For example- If my friend is late, so I might assume that he or she must have been stuck in traffic. So, this assumption is not tested yet and is made based on my observation. So, this is a hypothesis.
Whenever, a conclusion is given after several experiments and testing after observing something in science is referred as scientific theory.
For example- Atomic theory given by Dalton was based on several experiments. However, theories can also be proved wrong as Dalton's theory was proved wrong later on my JJ Thomson.
To know more about observation here
https://brainly.com/question/3608740
#SPJ1
An amateur entomologist captures a particularly excellent ladybug specimen in a plastic jar. The internal volume of the jar is 0.5L, and the air within the jar is initially at 1 atın. The bug-lover is so excited by the catch that he squeezes the jar fervently in his sweaty palm, compressing it such that the final pressure within the jar is 1.25 atm. What is the final volume of the ladybug's prison?
Answers
The final volume of the ladybug's prison is approximately 0.4 liters.
To determine the final volume of the ladybug's prison, we can use Boyle's Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature. The equation for Boyle's Law is:
P1 * V1 = P2 * V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
In this scenario, the initial volume (V1) is given as 0.5 L, and the initial pressure (P1) is 1 atm. The final pressure (P2) is 1.25 atm. We need to find the final volume (V2).
Plugging the given values into the equation, we have:
1 atm * 0.5 L = 1.25 atm * V2
Simplifying the equation, we find:
0.5 L = 1.25 atm * V2
Dividing both sides of the equation by 1.25 atm, we get:
0.5 L / 1.25 atm = V2
V2 ≈ 0.4 L
For such more questions on volume
https://brainly.com/question/31454001
#SPJ8
The average mass of all fluorine (F) atoms is 18.998 u.Do you think most fluorine atoms have 9 neutrons or 10 neutrons? Explain.
Answers
Answer:
10 neutrons
Explanation:
The mass of is made up of the total protons and neutrons in an atom.
Each particle has a mass of 1 amu (atomic mass unit).
All fluorine atoms have 9 protons.
If each proton is 1 amu, the protons must contribute 9 amu to the total mass of the atom (9 x 1 amu = 9 amu).
This means the neutrons must contribute a total mass of 10 amu (18.998 amu - 9 amu = ~10 amu).
If each neutron has a mass of 1 amu, there must be 10 neutrons in a fluorine atom (10 amu / 1 amu = 10 amu).
assume that a soil has a water content of 40 percent by weight and a bulk density of 1.3g per cubic centimeter. if the soil dries to 20 percent by weight and shrinks by an amount equal to the water loss, calculate the bulk density at 20 percent moisture
Answers
The bulk density at 20 percent moisture, given that the soil dries to 20 percent by weight is 1.4 g/cm³
How do i determine the bulk density?First, we shall obtain the initial volume of the soil. Details below:
Total mass = 100 gBulk density = 1.3 g/cm³Initial volume of soil =?Volume = mass / density
Initial volume of soil = 100 / 1.3
Initial volume of soil = 76.93 cm³
Next, we shall obtain the volume after the soil dries to 20 percent by weight. Details below:
We were told that the soil shrinks by an amount equal to the water loss, thus we have
Initial mass of water = 40% = 40 g
Water lost = 20% = 20 g
New mass of water = 40 - 20 = 20 g
Thus,
Volume = Equal value of mass of water lost = 20 cm³
Finally, we shall obtain the bulk density at 20 percent moisture. Details below:
Mass of dry soil = 60 gNew of mass water = 20 gTotal mass = 20 + 60 = 80 gramsInitial volume = 76.93 cm³Volume at 20 percent moisture = 20 cm³New volume = 76.93 - 20 = 56.93 cm³Bulk density at 20 percent moisture =?Bulk density at 20 percent moisture = Total mass / new volume
Bulk density at 20 percent moisture = 80 / 5.93
Bulk density at 20 percent moisture = 1.4 g/cm³
Learn more about bulk density:
https://brainly.com/question/32032726
#SPJ1
What is the pH of a solution with a hydrogen ion concentration of 1.3X10^-11?
-11.1
-0.11
-10.9
-1.3
Answers
Answer:
11,1
Explanation:
-log(1.3x10^-11)
The pH of a solution with a hydrogen ion concentration of 1.3X10^-11 is -11.1. Therefore, option A is correct.
What do you mean by the pH ?The potential of hydrogen ion concentration is referred to as pH. a way to gauge how basic or acidic a material is. The pH scale represents from 0 to 14.
A pH value of 7 is considered neutral on this scale, which implies it is neither acidic nor basic. It is more than acidic if the pH value is below seven, and more basic if the pH value is above seven.
The pH can control the presence of nutrients, biological processes, microbial activity, and chemical behaviour.
Given:
Hydrogen ion concentration = 1.3X10^-11
pH = -log [H+]
pH = -log(1.3x10^-11)
pH = -11.1
Thus,-11.1 is the pH of a solution with a hydrogen ion concentration of 1.3X10^-11, option A is correct.
To learn more about the pH, follow the link;
https://brainly.com/question/15289741
#SPJ6
Which equation shows an increase in entropy?
Hint: Look at the states of matter, g s l, of the chemicals in each equation. A C2H4(g) + H2(g) + C2H6(g) в Caco3(9) + Cao(s) - CO2(g) c Fe(s) + S (s) -+ FeS (s)

Answers
The equation C2H4(g) + H2(g) + C2H6(g) → Caco3(s) + Cao(s) + CO2(g) shows an increase in entropy due to the formation of a gas as a product. Option A
In this equation, the reactants on the left-hand side consist of gases (C2H4 and H2), while the products on the right-hand side include a solid (Caco3) and a gas (CO2).
When a reaction involves a change from gaseous to solid or liquid states, there is typically a decrease in entropy because the particles become more ordered and constrained in the solid or liquid phase.
Conversely, when a reaction involves the formation of gases, there is generally an increase in entropy because gases have higher degrees of molecular motion and greater freedom of movement compared to solids or liquids.
In the given equation, the reactants include three gaseous compounds (C2H4, H2, and C2H6), and one of the products is a gas (CO2). Therefore, the overall entropy of the system increases during this reaction.
The equation Fe(s) + S(s) → FeS(s) does not show an increase in entropy. Both the reactants (Fe and S) and the product (FeS) are solids. Since solids have lower entropy compared to gases or liquids, the entropy of the system does not increase in this reaction. Option A
For more such questions on entropy visit:
https://brainly.com/question/30481619
#SPJ8
when burning magnesium is lowered in a gas jar full of carbon 4 oxide it continued to burn explain
Answers
When burning magnesium is lowered in a gas jar full of carbon dioxide, the burning process continues because the carbon dioxide acts as an oxidizing agent.
What is a combustion reaction?A combustion reaction is a type of chemical reaction that occurs when a fuel, such as a hydrocarbon, reacts with an oxidizing agent, such as oxygen, to produce heat and light in the form of a flame. The basic equation for a combustion reaction is:
Fuel + Oxidant → Heat + Light + Products
In the case of burning magnesium, the fuel is magnesium and the oxidant is carbon dioxide. The products of the reaction are magnesium oxide and carbon dioxide. Combustion reactions are exothermic, meaning they release energy in the form of heat.
This means that the carbon dioxide provides the oxygen necessary for the combustion reaction to continue, allowing the magnesium to continue burning. Additionally, the carbon dioxide also helps to extinguish the flame by removing the heat and the oxygen from the combustion zone.
To know more about energy, visit:
https://brainly.com/question/1932868
#SPJ1
What did Josh do that allowed him to dissolve that much sugar in only 100 g of water?
Josh used sugar cubes instead of granulated sugar.
Josh used a pan with a large surface area.
Josh stirred the solution.
Josh heated the water.
Answers
Answer:
heated the water
Explanation:
which of the following nuclear equations has a correct characterization?
Answers
The correct answer is A.
The nuclear equation that correctly characterizes a nuclear reaction is one where the sum of the atomic numbers and the sum of the mass numbers on both sides of the equation are equal.
\(_{92}^{235}\textrm{U}+_{0}^{1}\textrm{n}\rightarrow _{54}^{140}\textrm{Xe}+_{38}^{94}\textrm{Sr}+3_{0}^{1}\textrm{n}\)
This conservation of both atomic and mass numbers ensures that the nuclear reaction obeys the laws of conservation of mass and conservation of charge.For example, consider the following nuclear equation:\(_{92}^{235}\textrm{U}+_{0}^{1}\textrm{n}\rightarrow _{54}^{140}\textrm{Xe}+_{38}^{94}\textrm{Sr}+3_{0}^{1}\textrm{n}\)
In this equation, the sum of the atomic numbers (92 + 0) and the sum of the mass numbers (235 + 1) on the left side are equal to the sum of the atomic numbers (54 + 38 + 0) and the sum of the mass numbers (140 + 94 + 3) on the right side. Therefore, this nuclear equation is correctly characterized and satisfies the conservation laws.The correct answer is A.
For such more question on nuclear equation
https://brainly.com/question/30743773
#SPJ8
Which of the following is an organic moleclue ?

Answers
Answer:
water
Explanation:
From what did Oceans form?
Answers
Answer:
God
Explanation:
How many grams of Cu are there in a sample of Cu that contains 1.09×1024 atoms?
Answers
1.15 g of Cu are there in a sample of Cu that contains\(1.09*10^{24}\)atoms
The number of grams of Cu in a sample containing\(1.09*10^{24}\)atoms can be determined using Avogadro's number, which is \(6.02*10^{23}\)atoms/mol.
First, we need to calculate the number of moles of Cu in the sample. To do this, we divide the number of atoms by Avogadro's number to get the number of moles:
\(\frac{1.09*10^{24} atoms }{6.02*10^{23} atoms/mol }= 1.81*10^{-1 }mol\)
The sample's mass needs to be determined next. Since copper has an atomic mass of 63.55 g/mol, we can use this to determine the sample's mass:
\(1.81*10^{-1} mol * 63.55 g/mol = 1.15 g\)
Therefore, the sample contains 1.15 g of Cu.
learn more about Avogadro's number refer:brainly.com/question/11907018
#SPJ1