Answers
The two properties which can be measured from the given information are streak and cleavage of the mineral.
What are minerals?Minerals are naturally forming inorganic substances having crystal structure and metallic characteristics. They have a definite chemical composition and homogeneity.
Streak is the color of powder form of mineral and cleavage is how the mineral cleave when we cut through a plane. Hardness says how much hard the solid is.
When the mineral breaks into cubes when struck with a hammer, we measure its cleavage properties. The red trail on scrapping indicates the streak of the mineral.
Hence, the two properties we can measure from breaking by hammer and scrapping by an unglazed porcelain are its cleavage and streak.
To learn more about minerals, refer the link below:
https://brainly.com/question/18078524
#SPJ2
Related Questions
how did corana virus start
Answers
Answer:
we know that it originally came from an animal, likely a bat. At this time, there is no evidence that animals play a significant role in spreading the virus that causesd
Explanation:
Answer:
THE VIRUS DIDNT COME FROM NO BAT!
Explanation:
THE VIRUS STARTED IN CHINA IN A LAB AND CHINA DIDNT EVEN TELL THE USA ABOUT IT THEY HIT IT FROM US. WHEN CHINESE PEOPLE WERE TRAVELING TO THE USA IT SPREADED.
The addition of chromic acid or chromate is a qualitative test for alcohols as the reaction causes a color change. However, not all alcohols react with chromic acid or chromate. Determine whether the named alcohol will react with chromic acid or chromate to cause a color change.
a. 3-hexanol
b. 1 -butanol
c. 2-pentanol
d. 3-ethyl-3-pentanol
Answers
Answer:
3-hexanol
1 -butanol
2-pentanol
Explanation:
Let us recall that chromic acid or chromate are strong oxidizing agents. When they are oxidized, their colour changes from orange to green.
This shows a reduction in chromic acid or chromate. The reaction of chromic acid or chromate with a primary alcohol yields a carboxylic acid while reaction with a secondary substrate yields an alkanal.
Note that Tertiary alkyl halides are not be oxidized hence reactions involving a point where invitation carried along occur.
3-ethyl-3-pentanol is a tertiary alkyl halide hence it can not be oxidized.
The diagram shows salt dissolved in water. What does it show about water molecules
and chloride ions?
+
Chloride
Oxygen
Hydrogen 4
Sodium
Answers
Answer:
that they all dissolved went on watef
What can happen if you completely fill a bottle with water and put it in a freezer?Give the bonds in play responsible for this effect and all the associated physico-chemical parameters
Answers
Answer: if we put a glass bottle completely filled with water in the freezer it can explode.
Explanation:
Most liquids contract as they are cooled, the molecules are moving slower, they can't defeat the intermolecular forces. These intermolecular forces become stronger and when they reach the freezing point they solidify. They are usually tightly packed in a crystalline structure.
Water has a weird behaviour. A water molecule is composed of two atoms of H and one atom of O. The great difference in electronegativity between H and O is what makes the molecule polar. And two molecules of water are attracted together because of what we call hydrogen bonding.
Hydrogen bonding is a special type of dipole-dipole attraction between molecules. This hydrogen bonding tendency gets stronger as the temperature gets lower.
When water freezes, its molecules get arranged in a cystalline structure that has a defined shape. The ice structure is completely hydrogen bonded, and these bonds force the crystalline structure to be very "open". This structure is less dense than liquid water (ice floats in liquid water). There are gaps between the molecules in the crystalline structure, the volume increases and the water expands.
One of the most common uses of gravimetric analysis is determining the concentration of an ion in a solution(i.e. to determine pollutants in a lake). If a sample of lake water turns out to have a concentration of (1.17x10^-2) M AgNO3 How many grams of NaCl are required to precipitate most of the Ag+ ions from (2.7x10^2) mL of the (1.17x10^-2) M AgNO3 solution? Assume the molar mass of NaCl is 58.45 g/mol.
Answers
Answer: 0.0185 grams of NaCI.
Explanation:
To determine the amount of NaCl required to precipitate most of the Ag+ ions from the given AgNO3 solution, we can use the following steps:
Determine the number of moles of Ag+ ions in the solution:
Molarity of AgNO3 solution = 1.17 x 10^-2 M
Volume of AgNO3 solution = 2.7 x 10^-2 L
Number of moles of Ag+ ions = Molarity x Volume
Number of moles of Ag+ ions = (1.17 x 10^-2) x (2.7 x 10^-2)
Number of moles of Ag+ ions = 3.159 x 10^-4 moles
Use the stoichiometry of the balanced chemical equation to determine the amount of NaCl required to precipitate most of the Ag+ ions:
AgNO3 (aq) + NaCl (aq) → AgCl (s) + NaNO3 (aq)
From the balanced chemical equation, we can see that 1 mole of AgNO3 reacts with 1 mole of NaCl to produce 1 mole of AgCl.
Therefore, the number of moles of NaCl required to precipitate most of the Ag+ ions is also 3.159 x 10^-4 moles.
Convert the moles of NaCl to grams:
Molar mass of NaCl = 58.45 g/mol
Mass of NaCl = Number of moles of NaCl x Molar mass of NaCl
Mass of NaCl = (3.159 x 10^-4) x 58.45
Mass of NaCl = 0.0185 grams
Therefore, 0.0185 grams of NaCl are required to precipitate most of the Ag+ ions from 2.7 x 10^-2 L of the 1.17 x 10^-2 M AgNO3 solution.
Bear in mind that this is not a rocket science. But it was fun. I enjoy the process. Thank you for asking.
Which activity is a model of thermal energy transferred by conduction. (Is science)

Answers
Answer:
Heat transferred between the electric burner of a stove and the bottom of a pain is transferred by conduction.
At a fixed temperature and pressure, a 0.474 mol sample of gas has a volume of 8.65 L. How many mol of gas will have a volume of 4.39 L under these same conditions?
Answers
The volume of the gas is directly proportional to the number of moles of the gas. Hence, when volume is reduced to 8.65 L to 4.39 L, number moles reduces to 0.24 mol.
What is Avogadro's law?According to Avogadro's law, the volume of a gas is directly proportional to the number of moles. Thus n/V = a constant
If n1 and V1 be the initial number of moles and volume and n2, V2 be the final quantities, then,
n1/V1 = n2/V2
Given, n1 = 0.474 mol
V1 = 8.65 L
V2 = 4.39 L
then, n2 = n1 V2/V1
n2 = (4.39 L× 0.474)/8.65 mol
= 0.24 mol
Therefore, the number of moles of gas at the reduced volume is 0.24 mol.
Find more on Avogadro law:
https://brainly.com/question/6590381
#SPJ1
How many mL of CH3OH are present in a 17.1 v/v% solution which also contains 320 mL of water?
Do not include units in your answer.
Answers
To calculate this, we need to understand that a 17.1 v/v% solution means that there are 17.1 mL of CH3OH in every 100 mL of solution.
Therefore, we can set up the following equation:
17.1 mL CH3OH / 100 mL solution = x mL CH3OH / (x mL CH3OH + 320 mL H2O)
Solving for x, we get:
x = (17.1/100) * (x + 320)
0.171x + 54.72 = x
0.829x = 54.72
x = 66.05 mL
However, we need to subtract the volume of water, so:
x - 320 = 66.05 - 320 = -253.95 mL
Since we can't have a negative volume, we know that there must have been an error in our calculations. This is because we can't have a solution with less than 0 mL of CH3OH.
Going back to the equation, we can see that the problem comes from assuming that the volume of the solution is 100 mL. Instead, we need to assume that there are 100 mL of the solution + 320 mL of water, for a total of 420 mL. The equation then becomes:
17.1 mL CH3OH / 100 mL solution = x mL CH3OH / 420 mL
Solving for x, we get:
x = (17.1/100) * 420
x = 71.82 mL
Subtracting the volume of water, we get:
71.82 mL - 320 mL = -248.18 mL
Again, this is negative. The error this time is due to assuming that the volume of the solution is 420 mL, when it's actually the sum of CH3OH and water volumes.
To correct this, we need to recognize that the volume of the solution is the sum of the CH3OH and water volumes. Using the equation above, we can solve for the volume of the solution:
17.1 mL CH3OH / 100 mL solution = x mL CH3OH / V mL solution
Solving for V, we get:
V = (x / 0.171) * (100 / 1)
V = 39.77 mL CH3OH
The volume of water is given as 320 mL. Therefore, the volume of the solution is:
V + 320 = 39.77 mL + 320 mL = 359.77 mL
Finally, we can use the volume percentage to calculate the amount of CH3OH in mL:
17.1 mL CH3OH / 100 mL solution = x mL CH3OH / 359.77 mL solution
Solving for x, we get:
x = (17.1/100) * 359.77
x = 61.54 mL
Therefore, there are 61.54 mL of CH3OH in the solution.
Iron is a metal. The structure of iron is described as a lattice of positive ions in a sea of
electrons. Which of the following statements about iron are correct?
1 iron conducts electricity because the electrons are free to move
2 iron has a high melting point due to the strong covalent bonds
3 iron is an alloy
4 iron is malleable because the layers of atoms can slide over one another
A. 1 only
B. 1 and 3
C. 1 and 4
D. 2, 3 and 4
Answers
Answer: 1and 4
Explanation: iron is an element not an alloy. An ionic lattice is not bonded covalently.
Please help I’m really stuck:/
1. Use the equation weight = mg to find the weight of a 45 kg child.
2. Find the speed of a caterpillar that crawls a distance of 6.0 cm every
2.0 seconds. The equation for speed is v=d/t.
3. The circumference of a circle equals 2 mr, where r is the radius. Find
the circumference of a compact disc that has a radius of 6.0 cm.
2
HOLT SCIENCE SPECTRUM
Answers
Answer:
i would say two
Explanation:
I did the math
Kidney stones generally form from the double displacement reaction of two salts that form an insoluble compound, a precipitate. (Read through the background for Week 1 for more information.) which of the following salts will participate in a double displacement reaction with Na3PO4 to form an insoluble compound(s): __________
Ca(NO3)2
(NH4)2SO4
NaCl
KNO3
Answers
Answer: \(Ca(NO_3)_2\)
Explanation:
A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
A double displacement reaction in which on e of the product is formed as a solid is called as precipitation reaction.
The balanced chemical equation for a precipitation reaction is:
\(2Na_3PO_4(aq)+3Ca(NO_3)_2(aq)\rightarrow Ca_3(PO_4)_2(s)+6NaNO_3(aq)\)
The salt that should be participated in the double displacement reaction should form the insoluble compound(s) i.e. Ca(NO3)2.
What is a double displacement reaction?It is the one where the ions should be exchanged. The salts should be soluble in water that should be designated by symbol and can be insoluble in water also same in the solid form. The same should be presented by (s) when the chemical formulas are mentioned. Also, it is one of the product that should be created known as the precipitation reaction.
Learn more about compound here: https://brainly.com/question/14700099
At a given temperature, the equilibrium constant for the formation of HI from H2 and I2 was found to be 29.9. Calculate the equilibrium constant for the decomposition of HI.
Answers
Answer:
The correct answer is: K'= 0.033.
Explanation:
The formation of HI from H₂ and I₂ is given by:
H₂ + I₂ → 2 HI K= 29.9
The decomposition of HI is the reverse reaction of the formation of HI:
2 HI → H₂ + I₂ K'
Thus, K' is the equilibrium constant for the reverse reaction of formation of HI. It is calculated as the reciprocal of the equilibrium constant of the forward reaction (K):
K' = 1/K = 1/(29.9)= 0.033
Therefore, the equilibrium constant for the decomposition of HI is K'= 0.033
Sodium hydrogen carbonate, on heating, produces sodium carbonate, water and carbon dioxide.
A recipe for chocolate chip cookies requires 1.5 dm' of carbon dioxide.
Calculate the mass of sodium hydrogen carbonate that should be used at R.T.P.
INa = 23; H = 1: C = 12; O = 16.]
[Note that 1 mole of gas occupies a volume of 24,000 cm at room temperature and pressure (RTP)]
Answers
The mass of sodium hydrogen carbonate : 10.5 g
Further explanationGiven
1.5 dm' of CO₂
1 mol gas= 24 L at RTP(25 °C, 1 atm)
Required
the mass of sodium hydrogen carbonate
Solution
Decomposition reaction of Sodium hydrogen carbonate :
2 NaHCO₃ (s) ⇒ Na₂ CO₃ (s) + H₂ O(g) + CO₂ (g)
mol CO₂ :
\(\tt mol=\dfrac{1.5}{24}=0.0625\)
From the equation, mol ratio of NaHCO₃ : CO₂ (g) = 2 : 1, so mol NaHCO₃ :
\(\tt 2\times 0.0625=0.125\)
Mass NaHCO₃(MW=23+1+12+3.16=84 g/mol) :
\(\tt mass=mol\times MW\\\\mass=0.125\times 84\\\\mass=10.5\)
The total energy of the car is its potential energy + its kinetic energy. This total energy remains constant across the length of the track. What would be the best way to increase the energy of the coaster as it proceeds through the course?
Responses
A Increase the height at point B. Increase the height at point B.
B Make the roller coaster longer. Make the roller coaster longer.
C Decrease the number of cars in each train. Decrease the number of cars in each train.
D Increase the height at point A.
Answers
The energy of the coaster can be increased through the course if we increase the height at point A.
What is the roller coaster?The roller coaster is a device that can be used to describe the conversion of energy from potential energy to kinetic energy. Let us note that the total energy of the system must always be constant and it is the sum of the kinetic energy and the potential energy.
Now we want to know how to increase the energy of the coaster as it proceeds through the course. This would have to do with an increase in the height of the point A.
Learn more about roller coaster:https://brainly.com/question/19920727
#SPJ1
When a solution is diluted with water, the ratio of the initial to final
volumes of solution is equal to the ratio of final to initial molarities
Select one:
True
-
Answers
The correct answer for this problem would be TRUE.
Explanation: it is true that when a solution is diluted with water, the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities.
When a solution is diluted with water, the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities. The statement is True.
Concentration refers to the amount of a substance in a defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution.
There are various methods of expressing the concentration of a solution.
Concentrations are usually expressed in terms of molarity, defined as the number of moles of solute in 1 L of solution.
Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the appropriate volume of a more concentrated solution (a stock solution) to the desired final volume.
Learn more about Concentrations, here:
https://brainly.com/question/10725862
#SPJ3
For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equilibrium constant expression is: _________
a. K = [ H+] [NO2-] / [HNO2]
b. K = [ H+] [N] [O]2 / [HNO2]
c. K = [ H+] [NO2-] / [HNO2]
d. K = [H+]2 [NO2-] / [HNO2]
e. None of these
Answers
Answer: For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equilibrium constant expression is K = [ H+] [NO2-] / [HNO2].
Explanation:
An expression that depicts the ratio of products and reactants raised to the power of their coefficients at equilibrium is called equilibrium constant.
An equilibrium constant is denoted by the symbol 'K'.
For example, the dissociation of nitrous acid in aqueous solution is as follows.
\(HNO_{2} \rightleftharpoons H^{+} + NO^{-}_{2}\)
Hence, its expression for equilibrium constant is as follows.
\(K = \frac{[H^{+}][NO^{-}_{2}]}{[HNO_{2}]}\)
Thus, we can conclude that for the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equilibrium constant expression is K = [ H+] [NO2-] / [HNO2].
a student investigated heat transfer using a bottle of water
Answers
The result of the Heat Transfer experiment is given as follows: "The molecule was increased in kinetic energy but in a random structure." (Option B)
What is Heat Transfer?Heat transfer is a thermal engineering subject that deals with the creation, consumption, conversion, and exchange of thermal energy across physical systems.
Heat transmission is categorized into several methods, including thermal conduction, thermal convection, thermal radiation, and energy transfer via phase shifts.
At 3 p.m., the water temperature is raised. The average kinetic energy of an item is related to its temperature. As a result, as temperature rises, so does average kinetic energy.
Kinetic energy is created by the random movement of molecules. Hence, the correct answer is "The molecule was increased in kinetic energy but in a random structure."
Learn more about Heat Transfer:
https://brainly.com/question/13433948
#SPJ1
Full Question:
A student investigated heat transfer using a bottle of water. The student placed the bottle in a room at 20.50C. The student measured the temperature of the water in the bottle at 7 a.m. and again at 3 p.m. The data from the investigation are shown in the table below.
[See attached image]
Question:How would you describe the average kinetic energy of the water molecules in the bottle at 7 a.m. to the average kinetic energy of the water molecules in the bottle at 3 p.m.
The molecules were increased in kinetic energy but in a uniform structure. The molecules were increased in kinetic energy but in a random structure. The molecules were decreased in kinetic energy but in a uniform structure. The molecules were decreased in kinetic energy but in a random structure.
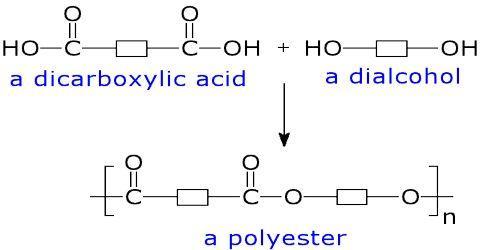
polyester is made by reacting which two chemicals?- acid and ester - acid and ketone - aldehyde and ketone- acid and alcohol
Answers
Let me explain this question using the following picture:
As you can notice, a polyester is formed when an acid and alcohol react together.
Therefore, the answer is acid and alcohol.
how do water molecules disslove in salt
Answers
Because the positive part of water molecules attracts negative chloride ions and the negative part of water molecules attracts positive sodium ions, water may dissolve salt. The solubility of a material is the quantity of that substance that can dissolve in a liquid (at a given temperature).
Why KHPo4 ignore effective as a buffer but kh2po4 is not
Answers
KH2PO4 is a more suitable choice as a buffer because it has a greater buffering capacity due to the presence of the weak acid and its conjugate base.
KHPo4 is not considered an effective buffer compared to KH2PO4 due to its limited buffering capacity. The effectiveness of a buffer is determined by the concentration and dissociation properties of its conjugate acid-base pair.
KH2PO4 is a salt composed of the weak acid H2PO4- and its conjugate base HPO4^2-. In an aqueous solution, KH2PO4 can dissociate to release H+ ions from the H2PO4- component, which acts as a weak acid, and the HPO4^2- component can accept H+ ions, acting as a weak base. This allows KH2PO4 to effectively resist changes in pH when small amounts of acid or base are added to the solution.
On the other hand, KHPo4 consists of the strong acid H3PO4 and the weak base HPO4^2-. H3PO4 fully dissociates in water, providing a large concentration of H+ ions, making it difficult for the HPO4^2- to effectively act as a base and maintain pH stability.
Therefore, KH2PO4 is a more suitable choice as a buffer because it has a greater buffering capacity due to the presence of the weak acid and its conjugate base.
For more question on conjugate
https://brainly.com/question/14684465
#SPJ8
What word means the same as Chordata?
A. non-vertebrate
B. vertebrate
Answers
n. urochordate, cephalochordate, tunicate, craniate, urochord, vertebrate.
What is the most similar element to an element with the atomic number of 113 and the outer energy level of 3?
Answers
The most similar element to an element is Nihonium.
What is a chemical element?A chemical element is a chemical substance that cannot be broken down into other substances. Atoms are the fundamental building blocks of chemical elements, and the number of protons in each atom's nucleus determines which chemical element it belongs to.
Furthermore, a substance whose atoms all contain the same number of protons is said to constitute an element; alternatively, all the atoms of a certain element must contain the same number of protons. Chemical reactions cannot degrade elements since they are the simplest chemical forms.
Learn more about chemical elements here:
https://brainly.com/question/28376204
#SPJ1
Suppose that you have 185 mL
of a buffer that is 0.420 M
in both formic acid (HCOOH)
and its conjugate base (HCOO−).
Calculate the maximum volume of 0.430M
HCl
that can be added to the buffer before its buffering capacity is lost
Answers
The buffer, which contains the salt solution of the weak acid formic acid and a strong base called $NaOH$, is called $HCOOH/HCOONa$.
In chemistry, what exactly is an acid?An acid is any substance that, when dissolved in water, tastes sour, turns blue litmus paper red, combines with some metals to release hydrogen, or reacts with bases to generate compounds that encourage chemical reactions.
In the body, what is acid?When the body's pH is out of balance due to a high quantity of acid, this condition is known as acidosis. It can result in serious health issues if the organs of elimination are unable to eliminate excess acid. Treatment of the underlying illness or condition can aid in reducing the body's acidity if it is a contributing factor in acidosis.
To know more about Acid visit:
https://brainly.com/question/29796621
#SPJ1
_______ occurs when a body’s molecular wavelength sends vibrations to another body, resulting in the production of another sound wave
Answers
Answer:
Natural frequency
Explanation:
Answer:
Resonance
Explanation:
Who among, Amit and Saaketh, is correct? Explain.
Answers
Answer:
What is their question answers exactly? I won't be able to explain who is correct.
Explanation:
Answer:
Not a lot to draw from this question if im going to be honest
Explanation
The mass number of Fe2+ is 56. How many neutrons are there in a single Fe2+ atom?
28
30
56
58
Answers
Answer:
A.) 28
Explanation:
The mass number is the number of protons and neutrons within a particular atom. The charge of the atom is irrelevant because the mass number is not affected by the number of electrons.
Unless this atom is an isotope (different number of neutrons), there should be an equal amount of protons and neutrons.
As such, since the mass number is 56, there should be 28 protons and 28 neutrons (56 / 2 = 28).
Does anyone know Chemistry
Answers
Answer:
so so
Explanation:
this your question?? <_>
Which statement is true?
a. Kinetic energy becomes potential energy in chemical reactions.
b. Energy can be created or destroyed, but matter cannot.
c. Matter and energy are conserved in chemical reactions.
d. Matter can be created or destroyed, but energy cannot.
Answers
Answer: c. Matter and energy are conserved in chemical reactions.
Explanation:
According to the law of conservation of matter, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side.
For every chemical reaction, the law of conservation of energy is applicable which states that the energy of the system remains conserved. Energy can neither be created nor destroyed. It can be transformed from one form to another.
Can somebody please help me!!!

Answers
Answer:
energy
Explanation:
Energy is used to do work or produce change. Friction and velocity do not do this.
Why is the combined gas law the most practiced gas law
Answers
The combined gas law allows you to derive any of the relationships needed by combining all of the changeable peices in the ideal gas law: namely pressure, temperature and volume.