a metal worker melts 65g of solid lead to make finish sinkers. calculate the energy change during melting. (thee heat of fusion of lead is 4.64kj/mol)
Answers
The energy change during melting the lead would be equal to the heat of fusion of lead which is 1.44 KJ for 65g.
Chemical element lead has the atomic number 82 and the letter Pb for its symbol. It is a dense hefty metal, unlike the majority of everyday materials.
Sample of Lead given : 65g
Heat of Fusion of lead : 4.64 kj/mol
1 mole of lead weighs : 207 g
Number of moles in 65g of lead : 65/207 = 0.31 moles
1 mole of lead releases energy : 4.64 kj
Heat of fusion for 0.31 moles of Lead : 0.31 * 4.64 kj
: 1.44 KJ
To know more about Heat of Fusion :
brainly.com/question/14053504
#SPJ4
Related Questions
Which of the following ionic compounds is named without using a Roman numeral?
Answers
because calcium has a charge of +2 and oxygen has a charge of -2 which makes them balanced
1. CaO
2. tin(IV) sulfide
3. VI
4. ammonium
5. Nothing is wrong with the formula.
6. Cu +
7. reactant
8. yield sign
enjoy ;)
why is time an independent variable
Answers
A mothball, composed of naphthalene (C10H8) has a mass of 1.86 g. How many naphthalene molecules does it contain? Express your answer in molecules to three significant figures.
Answers
Answer:
1.476 mol molecules
Explanation:
What is the name of this alkane? Two central carbons are bonded to C H 3 at each end, H below, and C H3 above the left carbon and H above the right carbon.

Answers
Answer:
the first alternative is the answer.
Explanation:
first u choose the longest chian. that is the horizontal chain. then u count the carbon number. then u see if there are alkyls the alkyl is methyl. and it is 1. so, you first write the alkyl number (1) then the alkyl name (methyl) then you write the alkane name (butane) because it consists 5 carbons. hope it helped.
The correct answer is 1-methyl butane.
What are alkanes?
Alkanes are a series of compounds that contain carbon and hydrogen atoms with single covalent bonds. These are known as saturated hydrocarbons. This group of compounds consists of carbon and hydrogen atoms with single covalent bonds.
Does 1 methyl butane exist?While writing the IUPAC name the chain with the highest number of C atoms is considered to be the parent chain and since 1-methyl butane; methyl also forms the part of the chain so we shall use pentane (normal pentane and not 1-methyl butane) so literally, pentane and 1-methyl butane is the same molecule and not isomers.
Learn more about alkanes here https://brainly.com/question/24270289
#SPJ2
The half-life of a reaction of the first order completes in 10 minutes. How much time will be needed for the 80% completion of this reaction?
Answers
A first-order reaction refers to a reaction in which the rate of the reaction is directly proportional to the concentration of a single reactant raised to the first power and is expressed as it would take approximately 46.4 minutes for the 80% completion of this first-order reaction to occur. 46.4 minutes.
According to the given information:Rate = k[A]
In this equation, k is the reaction rate constant, and [A] represents the concentration of reactant A.
The half-life of a reaction of the first order completes in 10 minutes. We need to find out how much time will be needed for the 80% completion of this reaction.
To solve for the time needed for 80% completion of a reaction of the first order, we need to use the formula:
Time for 80% completion = 2.303/k x log ([A]₀/[A]t)
where k is the reaction rate constant, [A]₀ is the initial concentration of the reactant and [A]t is the concentration of the reactant after the given time t, and 2.303 is a conversion factor.
Let [A]₀ = 1 and [A]t = 0.2 (since 80% completion means 20% of the original concentration remains)
We know that, t1/2 = 10 min;
therefore, k = 0.693/t1/2
= 0.693/10
= 0.0693 (as 0.693 = ln2)Now,
Time for 80% completion
= 2.303/k x log ([A]₀/[A]t)
= 2.303/0.0693 x log(1/0.2)
= 46.4 minutes
Therefore, it would take approximately 46.4 minutes for the 80% completion of this first-order reaction to occur. 46.4 minutes.
To know more about first-order reactions visit:
https://brainly.com/question/15909753
#SPJ11
write a balanced equation for the reaction of thiosulfate (s2o32-) and permanganate (mno4-) ions in acidic solution to form sulfate and manganese(ii) ions.
Answers
When potassium permanganate is reacted with thiosulfate, then there is formation of potassium sulfate and magnese ion.
What is potassium permanganate?The inorganic substance potassium permanganate has the formula KMnO₄. It is a crystalline salt that is purple-black and dissolves in water into a solution of K+ and MnO₄.
As a potent oxidizing agent, dermatitis medicine, wound cleaner, and general disinfectant, potassium permanganate is widely employed in the chemical industry and labs.
A balanced equation for the reaction of thiosulfate permanganate ions in acidic solution to form sulfate and manganese (ii) ions is
KMno₄ + Na₂S₂o₃ + H₂O ⇒ K₂So₄ + KOH + MnO₂ + Na₂So₄
Thus, the equation is KMno₄ + Na₂S₂o₃ + H₂O ⇒ K₂So₄ + KOH + MnO₂ + Na₂So₄.
To learn more about potassium permanganate, follow the link;
https://brainly.com/question/20630418
#SPJ1
when 0.325 mol of a weak acid, hx, is dissolved in 2.00 l of aqueous solution, the ph of the resultant solution is 2.76. calculate ka for hx.
Answers
The Ka of The pH of the resulting solution is 2.76 when 0.325 mol of the weak acid hx is dissolved in 2.00 l of water solution is 2.76 x \(10^{-3}\)
The Ka of the weak acid HX can be calculated using the following equation: Ka = [H+]2 / [HX]. Since the pH of the resultant solution is 2.76, [H+] = 10-2.76 = 0.00712. We can also calculate [HX] using the concentration of the initial acid, which was 0.325 mol.
Since the volume of the solution is 2.00 L, [HX] = 0.325 mol / 2.00 L = 0.1625 M. Substituting these values into the Ka equation gives us Ka = 0.007122 / 0.1625, which equals 2.76 x \(10^{-3}\)
Learn more about pH here:
https://brainly.com/question/26856926
#SPJ11
Alcohols can be dehydrated to give alkenes by treatment with POCl3 in the presence of pyridine. For the reaction below: Write a mechanism for the step below using curved arrows to show electron reorganization.
Answers
The step involving the dehydration of alcohols to form alkenes using POCl3 in the presence of pyridine can be explained through a mechanism that involves the formation of a cyclic intermediate and the subsequent elimination of water. Curved arrows are used to represent electron reorganization during the process.
The dehydration of alcohols using POCl3 and pyridine involves a nucleophilic substitution reaction. The mechanism begins with the lone pair of electrons on the oxygen atom of the alcohol attacking the electrophilic phosphorus atom in POCl3. This forms a new bond between the oxygen and phosphorus atoms, while the chlorine atom of POCl3 leaves as a chloride ion.
The resulting intermediate is a cyclic structure known as an oxocarbenium ion, where the positive charge is localized on the carbon atom that was originally bonded to the hydroxyl group of the alcohol. The oxygen atom retains a positive charge.
In the next step, the pyridine molecule, acting as a base, abstracts a proton from the carbon atom of the oxocarbenium ion. This deprotonation step results in the formation of a carbon-carbon double bond, or an alkene, as well as the regeneration of the pyridine base.
During the mechanism, it is important to show the movement of electrons using curved arrows. These arrows indicate the flow of electron pairs and help illustrate the electron reorganization that occurs during bond formation and bond-breaking steps.
Overall, the mechanism for the dehydration of alcohols using POCl3 and pyridine involves the formation of a cyclic intermediate (oxocarbenium ion) followed by the elimination of water to generate the desired alkene product.
Know more about nucleophilic substitution reaction here:
https://brainly.com/question/30633020
#SPJ11
What is the mass of 10.0 mol CH₂O2?
10.0 mol CH₂O2 46.03 g CH₂O₂
1 mole CH₂O₂
[?] g CH₂O₂
Answers
Answer:
460 g
Explanation:
We have been asked to determine the mass of 10 mol CH2O2.
We know that 1 mol CH2O2 = 46g.
Thus to find 10 mol CH2O2 we cross multiply as shown below;
mass = (10 mol x 46 g)/(1 mol)
= 460 g of CH2O2
Therefore the mass of 10 mol CH2O2 is 460 g
A 75.0 mL portion of a 1.60 M solution is diluted to a total volume of 278 mL. A 139 mL portion of that solution is diluted by adding 193 mL of water. What is the final concentration? Assume the volumes are additive.
Answers
In this question we have two dilutions occurring, for both cases we will use the dilution formula, which says that the initial concentration and volume of a solution must be equal to the final concentration and volume of the solution, we can better understand that formula presenting it to the question:
M1 * V1 = M2 * V2
where:
M1 = initial molar concentration
V1 = initial volume in liters
M2 = final molar concentration
V2 = final volume in liters
Now let's see what happens in our first dilution:
1.60 M * 0.075 L = M2 * 0.278 L
M2 = 0.43 M, this is the first final concentration
Now we have a solution with 0.43M and 278 mL
In the question we take 139 mL of the 0.43 M solution and add 193 mL, therefore having 332 mL as final volume, let's use the formula again
0.43 M * 0.139 L = M2 * 0.332 L
M2 = 0.180 M as final concentration
It takes 547 kJ to remove one mole of electrons from the atoms at the surface of a solid metal.
What is the maximum wavelength of light capable of doing this?
Answers
According to the relation of variables in the electromagnetic spectrum the maximum wavelength of light is 36.3 ×10\(^-\)³¹ m.
What is electromagnetic spectrum ?The electromagnetic spectrum consists of electromagnetic radiation consists of waves made up of electromagnetic field which are capable of propogating through space and carry the radiant electromagnetic energy.
The radiation are composed of electromagnetic waves which are synchronized oscillations of electric and magnetic fields . They are created due to change which is periodic in electric as well as magnetic fields.
In the given problem,energy is related to wavelength by the formula, λ=hc/E,λ=6.626×10\(^-34\)×3×10⁸/547×1000=36.3×10\(^-31\) m.
Thus, the maximum wavelength of light is 36.3×10\(^-31\) m.
Learn more about electromagnetic spectrum,here:
https://brainly.com/question/23727978
#SPJ1
Is oxygen a metal or a nonmetal? how many valence electrons does an oxygen atom have?
Answers
Oxygen is a nonmetal and it has 6 valence electrons. (2s subshell has two and 2p subshell has four)
why oxygen is not a metal ?The element's size shrinks as it moves from left to right in a period, while its electronegativity rises. Therefore, we can conclude that an element's non-metallic property grows as an element moves from left to right over time.Elements get bigger and have lower electronegativities as they move down the group. As a result, their ionization energy drops and they become more metallic.Therefore, if we look at oxygen, it is situated in the sixteenth group, which is to the right of the periodic table, and the second period, which is smaller in size than other elements.
As a result, we can infer that the oxygen atom will have a significant non-metallic character because of its tiny size and strong electronegativity.
what do you mean by valence electron ?The electrons in an atom's outermost shell are called valence electrons. Because the electrons in the outermost shells of two atoms are the first to come into contact with one another and are the ones that control how an atom will react in a chemical reaction, when two atoms interact.
learn more about oxygen atom visit :
https://brainly.com/question/19532510
#SPJ4
Answer:
Oxygen is a non metal, and has 6 valence electrons.
Explanation:
I did it on plato
Caffeine, a stimulant found in coffee and soda, hasthe mass percent composition: C. 49.48%, H, 5.19%. N. 28.85% 0. 16.48% The molar mass ofcaffeine is 194. 19 g/molFind the molecular formula of caffeine.Express your answer as a chemical formula
Answers
We have the next % composition:
C. 49.48%
H, 5.19%.
N. 28.85%
0. 16.48%
We assume 100 g of sample
1) As we have 100 g of sample of Caffeine, we calculate the mass of each element involved here.
C. 49.48 g
H, 5.19 g
N. 28.85 g
0. 16.48 g
2) We calculate the number of moles of each element (we need the mass per mole of each element)
For C) 12.01 g/mol
49.48 g x (1 mol/12.01 g) = 4.120 moles
For H) 1.007 g/mol
5.19 g x (1 mol/1.007 g) = 5.154 moles
For O) 15.99 g/mol
16.48 g x (1 mol/15.99 g) = 1.030 moles
For N) 14.00 g/mol
28.85 g x (1 mol/14.00 g) = 2.060 moles
3) We choose the smallest number from 2) and divide the rest of them by it.
For C) 4.120 moles/1.030 moles= 4
For H) 5.154 moles/1.030 moles= 5
For O) 1.030 moles/1.030 moles= 1
For N) 2.060 moles/1.030 moles= 2
4) The numbers in 3) represents the subindex from the empirical formula of caffeine:
\(C_4H_5O_1N_2\)5) We calculate the molar mass of our empirical formula, 97.06 g/mol.
We already have the molar mass of the molecular formula, so we proceed like this:
n= the molar mass of the molecular formula/the molar mass of the empirical formula
n = 194.19 g/mol/97.06 g/mol = 2 approx.
We use "n" and we multiply our empirical formula by n = 2:
Therefore, our molecular formula:
\(C_8H_{10}O_2N_4\)Why is chemistry important? i need 5 paragraph please
Answers
Answer:
Chemistry has a reputation for being a complicated and boring science, but for the most part, that reputation is undeserved. Fireworks and explosions are based on chemistry, so it's definitely not a boring science. If you take classes in chemistry, you'll apply math and logic, which can make studying chemistry a challenge if you are weak in those areas. However, anyone can understand the basics of how things work, and that's the study of chemistry. In a nutshell, the importance of chemistry is that it explains the world around you.
Chemistry Explained
Cooking: Chemistry explains how food changes as you cook it, how it rots, how to preserve food, how your body uses the food you eat, and how ingredients interact to make food.
Cleaning: Part of the importance of chemistry is it explains how cleaning works. You use chemistry to help decide what cleaner is best for dishes, laundry, yourself, and your home. You use chemistry when you use bleaches and disinfectants, even ordinary soap and water. How do they work? That's chemistry.
Medicine: You need to understand basic chemistry so you can understand how vitamins, supplements, and drugs can help or harm you. Part of the importance of chemistry lies in developing and testing new medical treatments and medicines.
Environmental Issues: Chemistry is at the heart of environmental issues. What makes one chemical a nutrient and another chemical a pollutant? How can you clean up the environment? What processes can produce the things you need without harming the environment?
We humans are all chemists. We use chemicals every day and perform chemical reactions without thinking much about them. Chemistry is important because everything you do is chemistry! Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything.
Importance of Taking Chemistry
Everyone can and should understand basic chemistry, but it may be important for you to take a course in chemistry or even make a career out of it. It's important to understand chemistry if you are studying any of the sciences because all of the sciences involve matter and the interactions between types of matter.
Students wanting to become doctors, nurses, physicists, nutritionists, geologists, pharmacists, and (of course) chemists all study chemistry. You might want to make a career out of chemistry because chemistry-related jobs are plentiful and high-paying. The importance of chemistry won't be diminished over time, so it will remain a promising career path.
Paragraph 1:
Chemistry is important Because it is so fundamental to our world, chemistry plays a role in everyone's lives and touches almost every aspect of our existence in some way.
Paragraph 2:
Chemistry is essential for meeting our basic needs of food, clothing, shelter, health, energy, and clean air, water, and soil.
Paragraph 3:
Even your body is made of chemicals. Chemical reactions occur when you breathe, eat, or just sit there reading. All matter is made of chemicals, so the importance of chemistry is that it's the study of everything.
Paragraph 4:
Chemistry has a reputation for being a complicated and boring science, but for the most part, that reputation is undeserved.
Paragraph 5:
Fireworks and explosions are based on chemistry, so it's definitely not a boring science. If you take classes in chemistry, you'll apply math and logic, which can make studying chemistry a challenge if you are weak in those areas.
Hope it helps :))
Name the substance added to the furnace to reduce the zinc oxide.
Answers
Answer:
Chemical was added in the zinc oxide to the theorems to reduce the zinc oxide.
Explanation:
The cultures of prehistoric humans are known mostly through the excavation of stone tools and other relatively imperishable artifacts. The early tool making traditions are often referred to as being paleolithic (literally "Old Stone Age). The Oldowan and Acheulian tool traditions of the first humans were the simplest applied research basic research Scientihe thought O philosophies technologies
Answers
The cultures of prehistoric humans are primarily known through the excavation of stone tools and other durable artifacts, such as the Oldowan and Acheulian tool traditions.
Stone tools and imperishable artifacts serve as key archaeological evidence for understanding prehistoric cultures. Through meticulous excavation and analysis, archaeologists have been able to piece together the lifestyles, technological advancements, and social behaviors of early human societies. The term "paleolithic" refers to the Old Stone Age, a time when humans relied on stone tools as their primary implements.
The Oldowan tool tradition is considered the earliest stone tool industry, dating back around 2.6 million years ago. It is characterized by simple tools, such as choppers and scrapers, which were crafted by flaking off pieces from larger stones. These tools were primarily used for basic activities like butchering and processing animal carcasses.
Later, the Acheulian tool tradition emerged around 1.76 million years ago, representing an advancement in stone tool technology. Acheulian tools, such as handaxes and cleavers, were more refined and standardized, showcasing an increased level of sophistication in tool-making techniques. These tools served a wide range of purposes, including hunting, woodworking, and shaping raw materials.
By studying the Oldowan and Acheulian tool traditions, researchers gain valuable insights into the cognitive abilities, cultural development, and technological progress of early humans. The examination of these artifacts provides evidence of their adaptability, problem-solving skills, and the gradual refinement of their tool-making techniques over time.
Learn more about prehistoric humans
brainly.com/question/28301954
#SPJ11
Balance the equation choose the coefficient for blank 3 (in front of KBr)___ KOH + ___ HBr --> ___ KBr + ___ H2O
Answers
Balancing the equation :
KOH(aq) + HBr(aq) --> KBr(aq) + H2O(l)
This is the balanced chemical reaction because it follows the following ionic reaction:
H ^(aq)+ + Br^(aq)- + K^+(aq) + OH ^- (aq) → K^+(aq) + Br^-(aq) + H2O (l)
What mass in grams of Na2S2O3 is needed to dissolve 4. 7 g of AgBr in a solution volume of 1. 0 L, given that Ksp for AgBr is 3. 3 x 10-13 and Kq for [Ag(S,O3)213- is 4. 7 x 1013? • Your answer should have two significant figures
Answers
A mass of 12.5 grams of \(Na_2S_2O_3\) is needed to dissolve 4.7 g of AgBr in a solution volume of 1 L.
The balanced equation for the dissolution of AgBr is:
AgBr (s) ↔ \(Ag^+\) (aq) + \(Br^-\) (aq)
The solubility product expression for AgBr is:
Ksp =\([Ag^+][Br^-]\)= 3.3 x \(10^{-13}\)
The reaction between \(Ag^+\) and \(S_2O_3^{2-}\) is:
\(Ag^+\) (aq) + 2 \(S_2O_3^{2-}\) (aq) ↔ \([Ag(S_2O_3)_2]^{3-}\) (aq)
The reaction quotient for \([Ag(S_2O_3)_2]^{3-}\) is:
Kq = [\(Ag^+\)]\([S_2O_3^{2-}]^2\) / \([Ag(S_2O_3)_2]^{3-}\) = 4.7 x \(10^{13}\)
We can use the solubility product expression to find the concentration of \(Ag^+\) in the solution:
[\(Ag^+\)] = Ksp / \([Br^-]\) = 3.3 x \(10^{-13}\) / (4.7 g / 187.77 g/mol / 1 L) = 1.64 x \(10^{-10}\)M
We can then use the reaction quotient to find the concentration of \(S_2O_3^{2-}\) in the solution:
\([S_2O_3^{2-}]\) = √(Kq \([Ag(S_2O_3)_2]^{3-}\) / \([Ag^+]\)) = √(4.7 x \(10^{13}\) / 1.64 x \(10^{-10}\)) / 2 = 7.9 x \(10^{-2}\) M
Finally, we can use the concentration of \(S_2O_3^{2-}\) to find the mass of \(Na_2S_2O_3\) needed to dissolve the AgBr:
mass = concentration x volume x molar mass = 7.9 x \(10^{-2}\) M x 1 L x 158.11 g/mol = 12.5 g
Learn more about the mass at
https://brainly.com/question/508865
#SPJ4
Identify an element on the periodic table that is chemically similar to neon (ne).
Answers
Plz answer these questions :

Answers
Answer: 2) see below
3) 2AgCl → 2Ag + Cl₂
Explanation:
2)
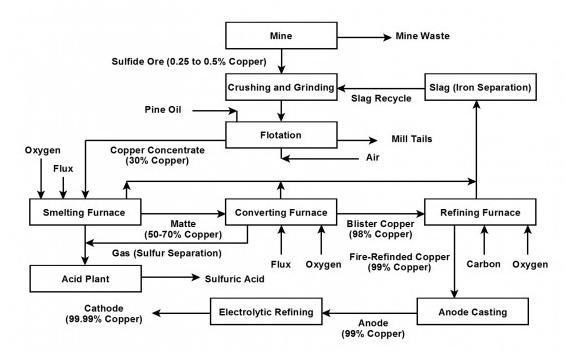
Step 1: Crushing & Grinding CuFeS₂(s)→ CuFeS₂(s)
Step 2: Froth Flotation CuFeS₂(s)----> CuFeS₂ (l)
Step 3: Roasting 2CuFeS₂(l) + 3O₂(g) → 2FeO(s) + 2CuS(s) + 2SO₂(g)
Step 4: Converting matte to blister Cu₂S(l) + O₂(g) → 2Cu(l) + SO₂(g)
Step 5: Anode Casting Cu(l) → Cu(s)
Step 6: Electrolytic Refining Cu(s) → Cu(s)
Anode: Cu(s) → Cu₂ + (aq) + 2e-
Cathode: Cu2 + (aq) + 2e- → Cu(s)
see diagram below for illustration of steps
3) When silver chloride (solid) is exposed to sunlight, it decomposes into silver (solid) and chlorine (gas).
The equation is written as: \(2AgCl_{(s)}\xrightarrow{\text{sunlight}}}2Ag_{(s)}+Cl_{2(g)}\)
what's going to be in the he World
Answers
Answer:
Lots of people set the alarm with the best of intentions, knowing that's the time they need to get up to meet the day's demands. But then the alarm clock seems to ring way before they're ready to rise, so they're hitting snooze and, eventually, running late. Something's got to give.
The key lies inside your body. "An important factor in being able to wake up easily at the desired time in the morning is the timing of one's circadian rhythm, or 'body clock,'" says sleep researcher Leon C. Lack, PhD, professor emeritus in the school of psychology at Flinders University in Adelaide, Australia. Much of what you need to do to wake up on time starts by planning your sleep schedule the day and the evening before — and by making your mornings count.
How do our internal clocks work, and how much can we control them? According to the National Institutes of General Medical Sciences (NIGMS), the body’s master clock, located in the brain, produces and regulates our circadian rhythms, which help determine sleep patterns over the course of a 24 hour period. Environmental signals, such as daylight and darkness, affect circadian rhythms, too. When incoming light hits the optic nerves, information is passed along from the eyes to the brain. When there is little or no light — at night — your clock tells the brain to make more melatonin, a hormone which makes you sleepy.
Our sleep-wake cycles, hormone levels, metabolism, and body temperature are all affected by our circadian rhythms, notes the National Institute of Neurological Disorders and Stroke. When your rhythm is off, you may be at risk for more than just a few groggy days you drag yourself through. Irregular rhythms, the NIGMS notes, have been linked to chronic health conditions, such as obesity, diabetes, depression, bipolar disorder, and seasonal affective disorder.
But there are ways to recalibrate your system to get the sleep you need and wake up feeling refreshed and ready for the day ahead. Physiological and psychological factors come into play, and it’s not always easy to get a good night’s rest or adhere to a schedule so that you consistently go to sleep and get up around the same time each day.
is going on
I need help asap!!! At least with the first part

Answers
Answer:
The correct answer -
a. Cd and Pb(NO3)2
b. Redox reactions
c. Pb and Cd(NO3)2
Explanation:
This is the reaction known as the redox or reduction-oxidation reaction of metals. In this particular reaction, there are two reactants Cadmium (III) in solid-state and lead (II) nitrate in the aqueous state. At the end of this reaction, the products that we get are lead (II) in solid-state and Cadmium (III) nitrate in the aqueous state.
cadmium (s)+ lead nitrate (aq) = lead (s) + cadmium nitrate (aq)
Cd (s) + Pb2+(aq) → Pb(s) + Cd2+(aq)
Here, Oxidizing agent is Pb2+ and the reducing agent is Cd.
Explain why the melting point of calcium is high?
Answers
Calcium has a high melting point which is about 842°C.
This is coz Calcium atoms have smaller radii since they have also a greater nuclear charge.
citric acid, the compound responsible for the sour taste of lemons, has the following elemental composition: cc, 37.51%%; hh, 4.20%%; oo, 58.29%%. calculate the empirical formula of citric acid.
Answers
The compound responsible for the sour taste of lemons, has the empirical formula C₆H₈O₇.
given that :
Carbon = 37.51 %
hydrogen = 4.20 %
oxygen = 58.29 %
moles of the carbon ,C = mass / molar mass
= 37.51 / 12
= 3.12 mol
moles of the hydrogen , H = 4.20 / 1
= 4.20 mol
moles of the oxygen , O = 58.29 / 16
= 3.64 mol
diving by the smallest one :
C = 3.12 / 3.12 = 1
H = 4.20 / 3.12 = 1.33
O = 58.29 / 3.12 = 1.17
by diving by the 6 we get : C₆H₈O₇
The empirical formula is C₆H₈O₇
To learn more about empirical formula here
https://brainly.com/question/14890059
#SPJ4
carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. what volume of sulfur dioxide would be produced by this reaction if of oxygen were consumed?
Answers
Volume of SO2 proced at the end of reaction = 19 mL
Data, carbon disulfide vol. = 9.5 mL
Since they are proportionate, we can use the vol-vol analysis if a reaction takes place in the gaseous phase exactly like the mole-mole analysis.
What is Ideal gas equation?
The macroscopic characteristics of ideal gases are related by the ideal gas law (PV = nRT). A gas is said to be perfect if its particles are both non-repellent and occupy no space (have no volume).
As per Ideal gas equation,
pv = nRT
and, v∝n
Therefore, to solve this question, we need to come with the eqution of the reaction.
Cs + 3O₂ → 2SO₂ + CO₂
9.5 - - - - Initial
0 - - - 2*9.5 9.5 after
Therefore, The Volume of SO2 proced at the end of reaction = 19 mL
To learn more about Ideal gas equation
Here: https://brainly.com/question/27870704
#SPJ4
At room temperature, compounds that are held together by covalent bonds tend to exist in what state?
Answers
at room temperature substances hawks together by covalent bonds are typically in liquid form
At room temperature substances hawks together by covalent bonds are typically in liquid or gas form.
What state are covalent bonds at room temperature?
Covalent compounds are typically liquids or gases at room temperature, although the more complex and the larger the molecule, the greater the chance that it could exist as a solid.
Which compound is held together by covalent bonds?Compounds that form from two or more nonmetallic elements, such as carbon and hydrogen, are called covalent compounds. In a covalent compound, atoms of the different elements are held together in molecules by covalent bonds. These are chemical bonds in which atoms share valence electrons.
Learn more about Covalent compounds here: brainly.com/question/11651796
#SPJ2
3.) A metal M forms a compound with the formula MC14. If the compound is 74.75 % chlorine, what is
the identity of M?
Answers
M = Iron, since the compound is 74.75% chlorine, the other 25.25% of the compound is iron. The compound is Iron(II) Chloride (FeCl2).
What is chlorine?Chlorine is a chemical element with the symbol Cl and atomic number 17. It is a yellow-green gas at room temperature and is the second-lightest halogen, and is a powerful oxidizing agent and disinfectant. Chlorine is used in a wide variety of applications, including water purification, bleaching, and many chemical manufacturing processes. Chlorine is also used to make chlorinated compounds, such as chloroform and chlorobenzene. In its elemental form, chlorine is a strong oxidizing agent, and it is used to disinfect drinking water and swimming pools, as well as to sterilize medical and laboratory equipment. Chlorine can also be used in bleaching wood pulp for paper production and for the production of many organic chemicals. Chlorine is also used in the manufacture of many food and pharmaceutical products.
To learn more about chlorine
https://brainly.com/question/24218286
#SPJ1
ASAP PLEASE! Mostly just need the data and conclusion answers please!!!
Electromagnetic Spectrum Lab Report
Instructions: In this virtual lab, you will use a virtual spectrometer to analyze astronomical bodies in space. Record your hypothesis and spectrometric results in the lab report below. You will submit your completed report to your instructor.
Name and Title:
Include your name, instructor's name, date, and name of lab.
Objectives(s):
In your own words, what is the purpose of this lab?
Hypothesis:
In this section, please include the predictions you developed during your lab activity. These statements reflect your predicted outcomes for the experiment.
Procedure:
The materials and procedures are listed in your virtual lab. You do not need to repeat them here. However, you should note if you experienced any errors or other factors that might affect your outcome. Using your summary questions at the end of your virtual lab activity, please clearly define the dependent and independent variables of the experiment.
Data:
Record the elements present in each unknown astronomical object. Be sure to indicate “yes” or “no” for each element.
Hydrogen Helium Lithium Sodium Carbon Nitrogen
Moon One
Moon Two
Planet One
Planet Two
Conclusion:
Your conclusion will include a summary of the lab results and an interpretation of the results. Please answer all questions in complete sentences using your own words.
Using two to three sentences, summarize what you investigated and observed in this lab.
Astronomers use a wide variety of technology to explore space and the electromagnetic spectrum; why do you believe it is essential to use many types of equipment when studying space?
If carbon was the most common element found in the moons and planets, what element is missing that would make them similar to Earth? Explain why. (Hint: Think about the carbon cycle.)
We know that the electromagnetic spectrum uses wavelengths and frequencies to determine a lot about outer space. How does it help us find out the make-up of stars?
Why might it be useful to determine the elements that a planet or moon is made up of?
Answers
Answer: This lab's goal is to investigate the absorbance patterns created by recently discovered moons and planets.
The first moon consists of Lithium and carbon, the second moon consists of sodium and nitrogen. Moving onto the planets, the first planet consists of hydrogen and carbon, and lastly, the second planet is consistent with helium and carbon.
How to explain the lab report
The theory was right; there have been no flaws in the outcome. The astronomical item observed by the spectrometer is the independent variable. The spectrum of any astronomical object is the dependent variable.
Space consists of bodies with different types of the electromagnetic spectrum. This includes high-energy bodies emitting radiation in short wavelengths and extremely short wavelengths such as in UV spectrum, X rays, and gamma rays. Conversely, other bodies might be emitting radiations in the longer wavelengths such as Microwaves and Radio waves.
The element missing from the moons and the planets would be Oxygen. It is to be remembered that Oxygen forms the base of the sustenance of life forms on Earth and forms an indispensable part of the carbon cycle. In the absence of oxygen, these planets and moons remain lifeless.
Stars emit heat and light. Along with the heat and light, radiations are emitted by the star. These radiations travel outward from stars and work as the signature of the stars. By analyzing the radiations from the stars, scientists back on Earth could deduce the physical conditions in the heart of a star including its constitution, temperature, and surface conditions.
The knowledge of the constitution of the elements making up the moon or planet is necessary to ascertain the life-sustaining capability of the same.
Answer: This lab's goal is to investigate the absorbance patterns created by recently discovered moons and planets.
The first moon consists of Lithium and carbon, the second moon consists of sodium and nitrogen. Moving onto the planets, the first planet consists of hydrogen and carbon, and lastly, the second planet is consistent with helium and carbon.
How to explain the lab report
The theory was right; there have been no flaws in the outcome. The astronomical item observed by the spectrometer is the independent variable. The spectrum of any astronomical object is the dependent variable.
Space consists of bodies with different types of the electromagnetic spectrum. This includes high-energy bodies emitting radiation in short wavelengths and extremely short wavelengths such as in UV spectrum, X rays, and gamma rays. Conversely, other bodies might be emitting radiations in the longer wavelengths such as Microwaves and Radio waves.
The element missing from the moons and the planets would be Oxygen. It is to be remembered that Oxygen forms the base of the sustenance of life forms on Earth and forms an indispensable part of the carbon cycle. In the absence of oxygen, these planets and moons remain lifeless.
Stars emit heat and light. Along with the heat and light, radiations are emitted by the star. These radiations travel outward from stars and work as the signature of the stars. By analyzing the radiations from the stars, scientists back on Earth could deduce the physical conditions in the heart of a star including its constitution, temperature, and surface conditions.
The knowledge of the constitution of the elements making up the moon or planet is necessary to ascertain the life-sustaining capability of the same.
give a reason why it is not advisable to heat magnesium before heating ammonium nitrate
Answers
Answer:
it can result in an increasing risk of the accumulation of decomposition products, self-heating (from the heat released by the slow decomposition reactions)
Magnesium can result in an increased risk of the accumulation of decomposition products, self-heating (from the heat released by the slow decomposition reactions)
What is ammonium nitrate?Ammonium nitrate is used commonly in fertilizers; in pyrotechniques, herbicides, and insecticides; and in the manufacture of nitrous oxide.
When ammonium nitrate is heated, it decomposes exothermically into nitrous oxide and water.
Hence, Magnesium can result in an increased risk of the accumulation of decomposition products, self-heating (from the heat released by the slow decomposition reactions)
Learn more about ammonium nitrate here:
https://brainly.com/question/5148461
#SPJ2
What is the positive and negative result of Baeyer's test?
Answers
Answer: A positive result is a cloudy yellow solution, or a yellow precipitate. A negative result is a clear, yellow, or orange solution with no precipitate