A metal, M , of atomic mass 56 amu reacts with chlorine to form a salt that can be represented as MClx. A boiling point elevation experiment is performed to determine the subscript , and therefore, the formula of the salt. A 30.2 g sample of the salt is dissolved in 100.0 g of water and the boiling point of the solution is found to be 376.81 K. Find the formula of the salt. Assume complete dissociation of the salt in solution.
CAN SOMEONE PLEASE HELP ME!!! I AM VERY CONFUSED AND ITS DUE TODAY BEFORE 11:55 THANK YOU IN ADVANCED
Answers
Answer:
MCl₂
Explanation:
The formula for boiling point elevation can be used to find x. The "complete dissociation" means there will be an ion of M and x ions of Cl in the solution. The number of moles of solute will be 30.2 grams divided by the molecular weight of MClx, where x is the variable we're trying to find.
\(\Delta T=imK_b\qquad\text{where i=ions/mole, m=molality, $K_b\approx 0.512$}\\\\376.81-373.15=(x+1)\dfrac{\text{moles}}{\text{kg solvent}}(0.512)\\\\\dfrac{3.66}{0.512}=(x+1)\dfrac{\dfrac{30.2}{56+35.45x}}{0.1}=\dfrac{302(x+1)}{56+35.45x}\\\\\dfrac{3.66}{0.512\cdot 302}(56+35.45x)=x+1\\\\\dfrac{3.66\cdot 56}{0.512\cdot 302}-1=x\left(1-\dfrac{3.66\cdot 35.45}{0.512\cdot 302}\right)\\\\x=\dfrac{50.336}{24.877}\approx 2.023\)
Then the formula for the salt is MCl₂.
Related Questions
Why need to add NaAlF6 to Al2O3?
Answers
So in the electrolytic reduction of alumina, cryolite is added along with fluorspar to–
– decrease melting point of alumina
– decrease viscosity of electrolyte (CaF
2is used) – increase conductivity
Hope this helps
How many grams of phosphorous is in the human skeleton if its average
weight is 11 kg and contains 58% of calcium phosphate (Ca3(PO4)2)?
Answers
hope this helps
I think most likely yes
Calculate the pH of a solution prepared by dissolving 2.35g of sodium acetate, CH3COONa, in 71.0 mL of 0.20 M acetic acid, CH3COOH(aq). Assume the volume change upon dissolving the sodium acetate is negligible. Ka of CH3COOH is 1.75 x 10^-5.
Answers
The pH of the solution prepared by dissolving 2.35g of sodium acetate, CH₃COONa, in 71.0 mL of 0.20 M acetic acid, CH₃COOH(aq) is 5.06
What is the pH of the solution?To find the pH of the solution, we need to use the equation for the acid dissociation constant, Ka:
Ka = [H+][CH₃COO⁻]/[CH₃COOH]We first solve for the concentration of H⁺:
[H⁺] = Ka * [CH₃COOH]/[CH₃COO⁻]
Substituting the values;
[H⁺] = (1.75 * 10⁻⁵) * (0.20) / [CH3COO-]
[H⁺] = 3.5 * 10⁻⁶ / [CH3COO-]
To find the concentration of CH₃COO⁻, we need to first find the number of moles of sodium acetate, CH₃COONa, that was dissolved:
moles of CH₃COONa = mass / molar mass
moles of CH₃COONa = 2.35 g / 82.03 g/mol
moles of CH₃COONa = 0.0287 mol
Since sodium acetate completely dissociates in water, the number of moles of CH3COO- produced is also 0.0287 mol.
Since the volume change upon dissolving the sodium acetate is negligible, we can assume the total volume of the solution is still 71.0 mL.
Now we can find the concentration of CH₃COO-:
[CH₃COO⁻] = moles / volume
[CH₃COO⁻] = 0.0287 mol / 0.0710 L
[CH₃COO⁻] = 0.404 M
Then [H⁺] will be:
[H⁺] = 3.5 * 10⁻⁶ / 0.404
[H⁺] = 8.66 * 10⁻⁶
Finally, the pH of the solution:
pH = -log (8.66 * 10⁻⁶)
pH = 5.06
Learn more about pH and Ka at: https://brainly.com/question/29047971
#SPJ1
For all the reactions, identify the reaction type and name the products formed.
1) Reaction of 2-methylpropan-1-ol with acidified potassium permanganate
2) Reaction of butan-2-ol with acidified potassium permanganate
3) Reaction of pentan-3-one with NaAlB4
4) Reaction between ethanol and ethanoic acid.
Answers
Answer:
Oxidation reaction; propanone and potassium manganate (VII) are formed.
Oxidation reaction; butan-2-one and potassium manganate (VII) are formed.
Reduction reaction; pentan-3-ol is formed.
Esterification reaction; ethyl ethanoate and water are formed.
Which force keeps planets in orbit around the Sun?
O A. Strong nuclear force
O B. Electric force
O C. Gravity
O D. Magnetic force
Answers
Answer: Gravity
Explanation:
A PE X
NEED ASAP!
What is the resolution of a monochromator, Δλeff, with a exit slit width of 500 micrometers and a \(D^{-1}\) of 1.8 nm/mm? Express the answer in nm.
Answers
The resolution of the monochromator is 3.6nm
What is a MonochromatorA monochromator is an optical device that is used to isolate a specific wavelength or range of wavelengths of light from a broader spectrum of light. It is typically used in spectroscopy, where the goal is to measure the intensity of light at a specific wavelength or over a range of wavelengths.
The resolution of a monochromator, Δλeff, is given by the equation: Δλeff = (D^-1) * (exit slit width)
Plugging in the given values:
Δλeff = (1.8 nm/mm) * (500 micrometers)
Converting micrometers to millimeters:
Δλeff = (1.8 nm/mm) * (0.5 mm)
Δλeff = 3.6 nm
Learn more on monochromator here;
https://brainly.com/question/917245
#SPJ1
Violet light has a wavelength of 4.50 x 10-12 m. What is the frequency?*
3 points
1.5 x10^20 1/s
0.0135 1/s
6.77x10^19 1/5
3x10^8 1/s
Green light has a frequency of 6.73 x 1014 1/s. What is the wavelength?*
3 points
2.24x10^6 m
4.46x10^-7 m
2.02 x 10^23 m.
4.95 x 10^-24 m
3 points
What is the energy (Joules) of violet light with a frequency = 3.43 x 10^14
1/s? *
4.54x10^-19 J
1.99x10^-25 J
1.93 x 10^-48 J
2.27 x 10^-19 J
Answers
Answer:
Frequency = 6.67x10¹⁹ s⁻¹
Wavelength = 4.46x10⁻⁷m
e = 2.27x10⁻¹⁹J
Explanation:
To convert frequency to wavelength and vice versa we use the equation:
Wavelength = Speed of light / Frequency
Speed ligth is 3x10⁸m/s
For a wavelength of 4.50x10⁻¹²m:
4.50x10⁻¹²m = 3x10⁸m/s / Frequency
Frequency = 3x10⁸m/s / 4.5x10⁻¹²m
Frequency = 6.67x10¹⁹ s⁻¹For a frequency of 6.73x10¹⁴s⁻¹:
Wavelength = 3x10⁸m/s / 6.73x10¹⁴s⁻¹
Wavelength = 4.46x10⁻⁷mAnd energy, e, from frequency, is obtained as follows:
e = h ₓ frequency
Where h is Planck's constant, 6.626x10⁻³⁴J*s
e = 6.626x10⁻³⁴J*s*3.43x10¹⁴s⁻¹
e = 2.27x10⁻¹⁹JA 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
Which substance has the highest ph?
A. Tomato
B. Unpolluted rainwater
C. Ammonium hydroxide
D. Orange juice
Answers
Answer:
c ammonium hydroxide nh4oh has higher ph value and its a base
plz mark me branliest
Answer:
c) ammonium hydroxide
Explanation:
ammonium hydroxide nh4oh has higher ph value, and it is an alkaline
Calculate the N/Z ratio for elements with atomic numbers 104 through 109. Are they in the belt of stability? Are they stable? How do you know?
Answers
The ratio of neutrons to protons, or the N/Z ratio, plays a crucial role in determining a nucleus' stability. The range of N/Z ratios in which nuclei are stable is generally referred to as the belt of stability.
How can you tell whether a substance is stable or unstable?If the forces between the constituents of the nucleus are equal, an atom is stable. If these forces are out of balance or if the nucleus has an excessive amount of internal energy, an atom is unstable (radioactive).
Z = 104 for Rutherfordium, element 104. The isotopes 261Rf and 262Rf, having masses of 261 and 262, respectively, have the longest half-lives. Accordingly, N/Z ratios are:
261Rf: N/Z = (261-104)/157 = 1.08
262Rf: N/Z = (262-104)/158 = 1.09
These N/Z ratios are a little bit higher than the average belt of stability values, which are about 1.0 for heavy nuclei. These isotopes are thought to be reasonably stable because they are close enough.
Z = 109 for Meitnerium, element 109. The isotopes 278Mt and 282Mt, with masses of 278 and 282, respectively, have the longest half-lives. Accordingly, N/Z ratios are:
278Mt: N/Z
To know more about neutrons visit:-
https://brainly.com/question/29248303
#SPJ1
how many elements are in Li2SO4?how many elements are in li2s 04 how many elements are in li2s 04
Answers
Answer:
3
Explanation:
the three elements involved in this compound are Li, S, O.
lithium, sulfur, and oxygen. Which create the ionic compound, "Lithium Sulfate."
7 atoms total, since there are two lithium, four oxygen, and one sulfate atom. this is a white inorganic salt.
There are three different types of elements in LiSO₄ which are lithium (Li), sulfur (S), and oxygen (O).
What is an element?
An element is a pure substance made of only one kind of atom which all have the same number of protons in their nuclei.
The chemical element was first presented by Robert Boyle who defined it as "incapable of decomposition”. Boyle’s this definition lies admirably close to present-day theory.
When different kinds of elements undergo particular chemical reactions then atoms are rearranged into new compounds connected together by chemical bonds.
All other naturally occurring elements occur on the Earth as compounds or mixtures. Air is a mixture of the elements nitrogen (N₂), oxygen (O), and argon (Ar), though it does contain carbon dioxide and water.
Given compound, lithium sulfate is formed of LiSO₄ has elements lithium, sulfur, and oxygen.
Learn more about the chemical element, here:
https://brainly.com/question/9249660
#SPJ2
The tomato is dropped. What is the velocity, v
, of the tomato when it hits the ground? Assume 86.0 %
of the work done in Part A is transferred to kinetic energy, E
, by the time the tomato hits the ground.
Express your answer with the appropriate units.
Answers
To determine the tomato's velocity when it hits the ground, we need more information. Specifically, we need the height from which the tomato was dropped and the tomato mass.
Without these details, it is impossible to calculate velocity accurately. The velocity of an object when it hits the ground depends on factors such as the height of the fall, the mass of the object, and any forces acting on it during the fall (such as air resistance).
If you can provide the necessary information, I can help you calculate the velocity of the tomato when it hits the ground.
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
I need help!!!!!!!!!!!!!!!!
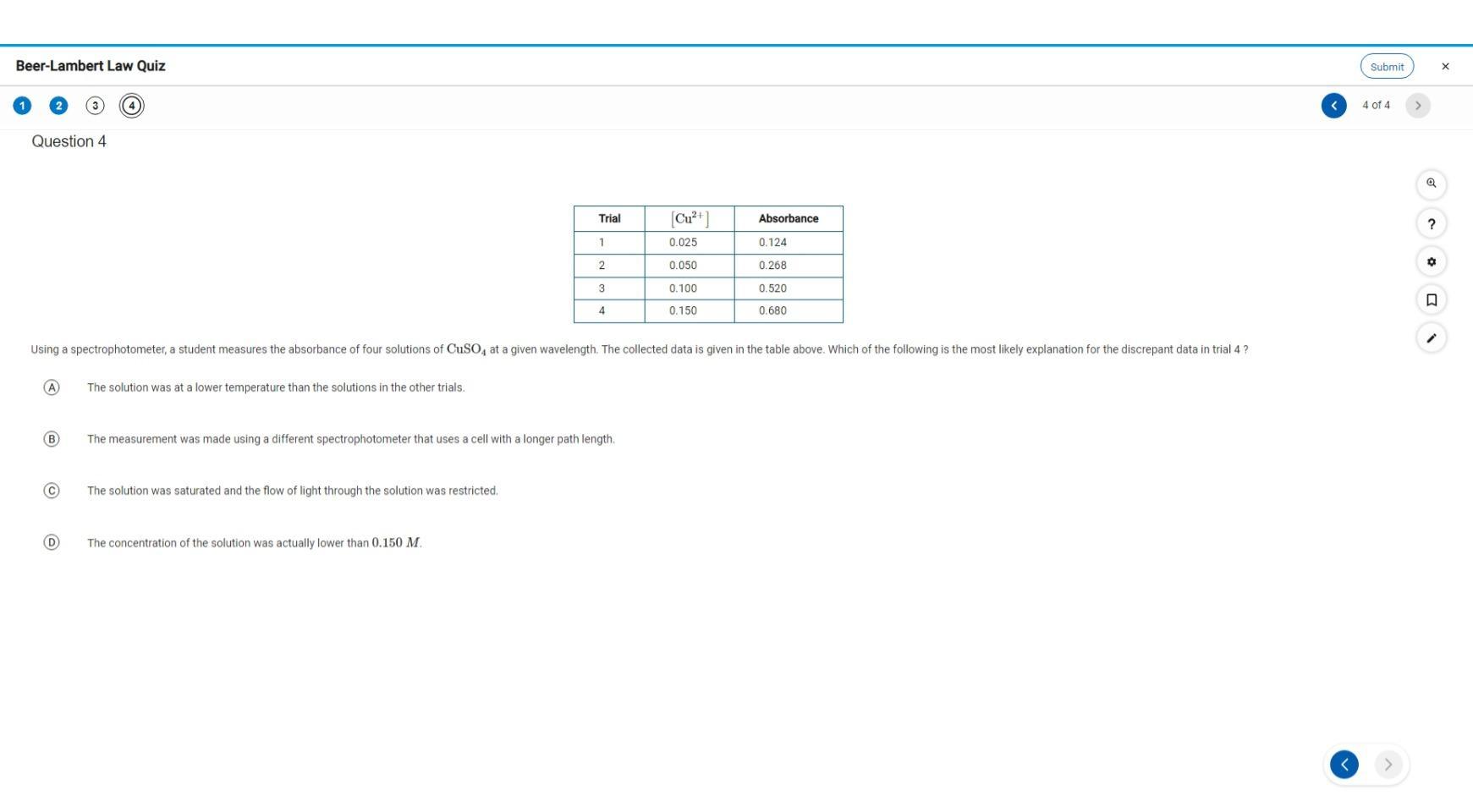
Answers
The reason for the fourth measurement is that the solution is saturated. Option C in the question.
What is the Beer Lambert's law?According to the Beer Lambert's law, the absorbance of the solution is found to the proportional to the concentration of the solution. This implies that as the concentration of the solution increases, the absorbance of the solution also increases.
The absorbance is proportional to the concentration, and the pathlength of the cell where the measurement is taken.
We can now see from the table that the absorbance of the solution is largely affected by the concentration of the solution and not really the pathlength.
Learn more about Beer Lambert's law:https://brainly.com/question/8831959
#SPJ1
A sample of 4.10 g of methane occupies 10.8 dm3 at 310 K. (a) Calculate the work done when the gas expands isothermally against a constant external pressure of 200 Torr until its volume has increased by 3.0 dm3.
Answers
Answer:
Explanation:
10.8 dm³ = 10.8 L
4.1 g of methane = 4.1 / 16 = .25625 moles
V₁ = 10.8 dm³
V₂ = 13.8 dm³
work done in isothermal expansion = 2.303 nRT log V₂ / V₁
= 2.303 x .25625 x 8.31 x 310 x log 13.8 / 10.8
= 1520.27 x log 1.2777
= 161.8 J.
please helpppppppppp
Answers
Answer:
Environmental factors such as diet, temperature, oxygen levels, humidity, light cycles
And the presence of mutagens can all impact which of an animal's genes are expressed, which ultimately affects the animal's phenotype.
Until a train is a safe distance from the station it must travel at 5 m/s Once the train is on open track it can speed up
to 45 mls. If it takes a train 8 seconds to reach 45 m/s, what is the acceleration of the train? (Round your answer to
the nearest whole number)
4 m/s2
5 m/s2
6 m/s2
7 m's?

Answers
Answer:
5 m/s²
Explanation:
From the question given above, it took 8 s for the train to get to a speed of 45 m/s. This simply means the train was maintaining 5 m/s as it travels in order to get to an open track.
Thus, we obtained the following data from the question.
Initial velocity (u) = 5 m/s
Final velocity (v) = 45 m/s
Time (t) = 8 s
Acceleration (a) =.?
Acceleration is simply defined as the rate of change of velocity with time. Mathematically, it can be expressed as:
a = (v – u) /t
Where:
a is the acceleration.
v is the final velocity.
u is the initial velocity.
t is the time.
With the above formula, we can obtain the velocity of the train as follow:
Initial velocity (u) = 5 m/s
Final velocity (v) = 45 m/s
Time (t) = 8 s
Acceleration (a) =.?
a = (v – u) /t
a = (45 – 5) / 8
a = 40/8
a = 5 m/s²
Therefore, the acceleration of the train is 5 m/s².
Answer:
Its B =)
Explanation:
How much volume of the above stock solution you will need to prepare the riboflavin solutions of following concentrations: (a) 0.059 mM in 25 ml (b) 14 uM in 25 ml
Answers
0.013 g of the stock solution of riboflavin needs to be added to 25 ml of water to prepare a 14 μM solution.
To prepare a 0.059 mM riboflavin solution in 25 ml, we need to calculate the amount of riboflavin to add to 25 ml of water.
First, we need to convert the concentration from mM to μM:
0.059 mM × 1000 μM/1 mM = 59 μM
Next, we need to calculate the amount of riboflavin required to prepare a 25 ml solution with a concentration of 59 μM:
59 μM × 25 ml = 1475 μmol
Finally, we need to convert μmol to milligrams:
1475 μmol × (375.2 g/mol) / (10⁻⁶ μmol/mol) = 0.055 g
So, 0.055 g of the stock solution of riboflavin needs to be added to 25 ml of water to prepare a 0.059 mM solution.
To prepare a 14 μM riboflavin solution in 25 ml, we need to calculate the amount of riboflavin to add to 25 ml of water:
14 μM × 25 ml = 350 μmol
Finally, we need to convert μmol to milligrams:
350 μmol × (375.2 g/mol) / (10⁻⁶ μmol/mol) = 0.013 g
So, 0.013 g of the stock solution of riboflavin needs to be added to 25 ml of water to prepare a 14 μM solution.
To know more about stock solution here
https://brainly.com/question/28083950
#SPJ4
Which of the following is true according the the Arrhenius theory of acids?
A strong acid increases the OH-ion concentration in an aqueous solution.
A strong acid decreases the OH-ion concentration in an aqueous solution.
O A strong acid decreases the H+ and OH-ion concentration in an aqueous
solution.
A strong acid increases the H+ ion concentration in an aqueous solution.

Answers
For example
HCl———>H+ + Cl-
what does the roman numeral stand for in copper(1) oxide should it not be copper(II) oxide
Answers
Answer:
The roman numeral in copper(I) oxide indicates that the oxidation number of copper in the compound is 1.
Explanation:
Roman numeral is used to indicate the oxidation number of an element in a compound.
The roman numeral in copper(I) oxide indicates that the oxidation number of copper in the compound is 1.
This can be seen from the following illustration:
copper(I) oxide => Cu₂O
Oxidation number of O = –2
Oxidation number of Cu₂O = 0
Oxidation number of Cu =?
Cu₂O = 0
2Cu + O = 0
2Cu – 2 = 0
Collect like terms
2Cu = 0 + 2
2Cu = 2
Divide both side by 2
Cu = 2/2
Cu = 1
Thus, we can see that the oxidation number of Cu in Cu₂O is 1. Hence the name of Cu₂O is copper(I) oxide indicating that the oxidation number of of copper (Cu) in the compound is 1.
For copper(II) oxide, we shall determine the oxidation number of Cu. This can be obtained as follow:
copper(II) oxide, CuO => CuO
Oxidation number of O = –2
Oxidation number of CuO = 0
Oxidation number of Cu =?
CuO = 0
Cu + O = 0
Cu – 2 = 0
Collect like terms
Cu = 0 + 2
Cu = 2
Thus, the oxidation number of Cu in CuO is 2. Hence the name of CuO is copper(II) oxide indicating that the oxidation number of of copper (Cu) in the compound is 2.
From the above illustrations,
We can see that the roman numeral in both copper(I) oxide, Cu₂O and copper(II) oxide, CuO are different because the oxidation number of Cu in both cases are different.
across the period the size of an atom decreases and effective nuclear charge
Answers
Answer:
Atomic radius decreases across a period and increases down a group. Across a period, effective nuclear charge increases as electron shielding remains constant. Down a group, the number of energy levels (n) increases, so there is a greater distance between the nucleus and the outermost orbital.
Explanation:
cool air tends to...
A. be less dense and flow over warm air.
B.be lifted up by more dense air
C.be more dense and flow under warm
D. mix easily with warm air masses
20 POINTS!!!
Answers
C. flow under dense and become more thick.
What does the chemical term "dense" mean?A substance that is tightly packed or has a high density.
The term "density" refers to the relationship between a substance's mass and the volume it takes up in space (volume). The mass, size, and arrangement of an object's atoms influence its density. The ratio of a substance's mass to its volume is said to be its density, or D.
Why does chemistry consider density?Because it enables us to predict which compounds will float and which will sink in a liquid, density is a crucial notion. An object will frequently float as long as its density is less than that of the liquid.
To know more about dense visit:
https://brainly.com/question/26364788
#SPJ1
How does the burning of fossil fuels contribute to global warming?
Answers
Answer: The burning of fossil fuels releases carbon dioxide, methane and other greenhouse gases into the atmosphere. These gases trap heat in the atmosphere, leading to a gradual increase in global average temperatures, known as global warming. This phenomenon has serious impacts on our environment and ecosystems, including extreme weather events and rising sea levels.
What kitchen products would you be able to replace without using petroleum made products?
Answers
Answer:
I would say maybe a stove? only if it's a gas stove tho- because of course you would need gas for a gas stove-
Becca is a forensic technician analyzing the fragments of a window. She sees that there is a hole in the window, and that the outside hole is smaller than the inside hole. What might she deduce from this information?
Answers
The observation of a smaller outside hole than inside leads Becca to infer that an impact from the outside caused the hole, with a larger object striking and passing through the window from the inside.
From the observation that the hole in the window is smaller on the outside than on the inside, Becca, as a forensic technician, might deduce the following:
The hole was caused by an impact from the outside: The smaller outside hole suggests that the force that created the hole originated from the outside and exerted more pressure on the window surface facing inward.
The object causing the hole was larger on the inside: The discrepancy in hole sizes implies that the object that struck the window had a larger size or diameter on the inside, and as it penetrated the glass, it compressed or fragmented the glass, resulting in a larger hole on the inside.
The object may have passed through the window: The difference in hole sizes indicates that the object may have penetrated the window, potentially passing through to the inside. This could suggest a break-in or an incident involving the window being struck from the outside.
Overall, the observation of a smaller outside hole than inside leads Becca to infer that an impact from the outside caused the hole, with a larger object striking and passing through the window from the inside.
For more question on observation
https://brainly.com/question/29521469
#SPJ8
Nonane and 2,3,4-trifluoropentane have almost identical molar masses, but nonane has a significantly higher boiling point. Which of the following statements best helps explain this observation?
Answers
Compared to 2,3,4-trifluoropentane, the nonane's carbon chains are longer.
In chemistry, what exactly is a molar mass?A substance's molar mass is defined as its molecular weight in grams. By adding the molar masses of a substance's constituent atoms, we may get the substance's molar mass. Then, to convert between mass and the quantity of moles of the material, we may utilize the computed molar mass.
A molar mass is determined in what way?Adding the atomic masses of a particular substance results in the calculation of molar mass. Below each element's symbol on the periodic table is a designation of the mass of that specific element. The molar mass is obtained by averaging the atomic masses obtained from the periodic table.
To know more about Molar mass visit:
https://brainly.com/question/22997914
#SPJ1
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
what are ambident nucleopliles
Answers
Answer:
I hope this helps you
.......................................

Calculate the cell potential for the galvanic cell in which the given reaction occurs at 25 °C, given that [Sn2+]=0.0624 M, [Fe3+]=0.0437 M, [Sn4+]=0.00655 M, and [Fe2+]=0.01139 M. Standard reduction potentials can be found in this table.
Sn2+(aq)+2Fe3+(aq)↽−−⇀ Sn4+(aq)+2Fe2+(aq)
So far my incorrect answers have been:
0.28
0.798
0.178
0.142
0.881
0.61
and 0.812
Answers
Answer:
The cell potential for the given galvanic cell is 0.188 V.
Explanation:
To calculate the cell potential, we can use the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
where E°cell is the standard cell potential, R is the gas constant (8.314 J/mol·K), T is the temperature in Kelvin (25°C = 298 K), n is the number of moles of electrons transferred (in this case, n = 2), F is the Faraday constant (96,485 C/mol), and Q is the reaction quotient.
First, we need to write the half-reactions and their standard reduction potentials:
Sn4+(aq) + 2e- → Sn2+(aq) E°red = 0.15 V
Fe3+(aq) + e- → Fe2+(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn2+(aq) + 2Fe3+(aq) → Sn4+(aq) + 2Fe2+(aq)
The reaction quotient Q can be expressed as:
Q = [Sn4+][Fe2+]^2 / [Sn2+][Fe3+]^2
Substituting the given concentrations, we get:
Q = (0.00655)(0.01139)^2 / (0.0624)(0.0437)^2 = 0.209
Now we can calculate the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe2+]^2/[Fe3+]) + 0.0592 V log([Sn4+]/[Sn2+])
= 0.15 V + 0.0592 V log(0.01139^2/0.0437^2) + 0.0592 V log(0.00655/0.0624)
= 0.188 V
Therefore, the cell potential for the given galvanic cell is 0.188 V.
The cell potential for the given galvanic cell in which the given reaction occurs at 25 °C is 0.188 V.
How to the cell potential of galvanic cell?To find the cell potential, we take the Nernst equation:
Ecell = E°cell - (RT/nF)ln(Q)
In which R is the gas constant (8.314 J/mol·K) and E° cell is the standard cell potential.
T temperature in Kelvin (25°C = 298 K), and n is the number of moles of electrons transferred (n = 2), Q is the reaction quotient and F is the Faraday constant (96,485 C/mol).
Firstly, write the half-reactions and then their standard reduction potentials:
Sn⁴⁺(aq) + 2e⁻ → Sn²⁺(aq) E°red = 0.15 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E°red = 0.77 V
The overall reaction is the sum of the half-reactions:
Sn²⁺(aq) + 2Fe³⁺(aq) → Sn⁴⁺(aq) + 2Fe²⁺(aq)
The Q reaction quotient can be written as:
Q = [Sn⁴⁺][Fe²⁺]² ÷ [Sn²⁺][Fe²⁺]²
Substituting the given concentrations, we observe:
Q = (0.00655)(0.01139)² ÷ (0.0624)(0.0437)² = 0.209
Next, we can find the cell potential:
Ecell = 0.15 V + 0.0592 V log([Fe²⁺]²/[Fe³⁺]) + 0.0592 V log([Sn⁴⁺]/[Sn²⁺])
= 0.15 V + 0.0592 V log(0.01139²÷0.0437²) + 0.0592 V log(0.00655÷0.0624)
= 0.188 V
Thus, the cell potential for the given galvanic cell is 0.188 V.
Learn more about cell potential, here:
https://brainly.com/question/29719917
#SPJ2