A mathematical equation useful for dilution calculations is Mdil × Vdil = Mconc × Vconc (a) What does each symbol mean, and why does the equation work?
Answers
Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water.” Diluting a solution entails adding more solvent without adding more solute.
How do you do dilution in math?The number of dilutions is equal to the number of times the dilution factor will be multiplied by itself to equal the starting concentration divided by the final concentration. So with a dilution factor of 10, 10 to the X power is equal to the starting concentration divided by the final concentration.
How do you find the dilution of a solution?You can solve for the concentration or volume of the concentrated or dilute solution using the equation: M1V1 = M2V2, where M1 is the concentration in molarity (moles/Liters) of the concentrated solution, V2 is the volume of the concentrated solution, M2 is the concentration in molarity of the dilute solution.
Learn more about dilution here:
https://brainly.com/question/1553819#SPJ4Related Questions
I need this turned in soon

Answers
Answer:
Explanation:
An element's atomic number defines the amount of protons an element may contain. The atomic number is usually the big number that is shown on top. Therefore, with 17 protons it will always be chlorine.
the indicated gene codes for a protein made up of the amino acid
Answers
Answer:
N/A
Explanation:
Is this a true or false answer or.....?
What number is pointing to the area where magma is less dense due to
heat?

Answers
Answer:
I think it's 2
Explanation:
The magma gets so hot, it floats up before cooling and sinking again
what is the solute of benzene.
Answers
Answer:Benzene is one of the ost commonly known aromatic compounds.Benzene is a solvent so they is no solute for it.
Explanation:mole fraction of solute in benzene is 0.5
Why does the angle of the sun at noon, seem to change at different moths throughout the year?
Answers
Answer:
because during different seasons the earth is closer or farther from the sun
Explanation:
the earths rotation around the sun is an oval with the winter season being the closest to the sun and the summer season being the farthest
What impacts do you think the Burmese pythons might have on local ecosystems?
Answers
Every impact in this world
Convert 48 g O2 to moles of O2
Answers
Answer:
1.5 moles
Explanation:
O mole weight = 15.999
O2 mole weight = 2 * 15.999 = 31.998 gm
48 / 31. 998 = ~ 1.5 moles
if a 1.00 ml sample of the reaction mixture for the equilibrium constant experiment required 32.40 ml of 0.258 m naoh to titrate it, what is the monoprotic acetic acid concentration in the mixture? select one: 0.399 m 5.82 m 8.36 m 7.96 m 12.6 m
Answers
The mixture contains a concentration of monoprotic acetic acid of
(8.36 M). (0.0083592 mol/1000 mL = 8.36 M) = 0.258 M x 32.40 mL/1000 L.
What is an example of monoprotic acid?Monoprotic acids include benzoic acid (C6H5CO2H), acetic acid (CH3CO2H or HOAc), nitric acid (HNO3), and hydrochloric acid (HCl).
What strength of acid is monoprotic acid?H2SO4 and H3PO4 are examples of polyprotic acids that contain two or three hydrogen ions. Although it is alluring to believe that polyprotic acids are more powerful than monoprotic acids since they include several hydrogen ions, this is not the case.
Learn more about hydrogen ion here:
https://brainly.com/question/7641960
#SPJ4
An atom has 15 protons, 15 electrons and 16 neutrons. What is the atomic
number and mass number?
Answers
Atomic number = 15 and mass number = 31 where an atom has 15 protons, 15 electrons and 16 neutrons.
What is an atom?An atom consists of a central nucleus that is usually surrounded by one or more electrons.
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. Atomic number = Number of protons.
Mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom.
Hence, atomic number=15 and mass number=31.
Learn more about the atom here;
https://brainly.com/question/4186338
#SPJ2
lonic compounds can conduct electricity in
Answers
Answer:
Ionic compounds conduct electricity when molten (liquid) or in aqueous solution (dissolved in water), because their ions are free to move from place to place. Ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move
Explanation:
Do you think a battery system has energy? Explain why.
Answers
Answer:
A battery is a device that stores chemical energy and converts it to electrical energy. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The flow of electrons provides an electric current that can be used to do work.
Explanation:
Which element has the highest electronegativity?
Answers
Answer:
fluorine
Explanation:
Answer:
The element with the highest electronegativity is fluorine with a score of 4.0 (which is the highest possible)
Explanation:
I personally haven't gotten this question yet so I hope this helps you!
Which two of the minerals shown have a metallic luster?

Answers
Answer:
A and D
Explanation:
Answer:
A & D
Explanation:
Convert 6.24 x 10g to standard notation.
Answers
Answer:
3.45 x 10^5
Explanation:
A ______ fatty acid has 2 or more locations in the carbon chain that are not saturated with hydrogen.
Answers
A polysaturated fatty acid has 2 or more locations in the carbon chain that are not saturated with hydrogen .
What is polyunsaturated fatty acid ?Polyunsaturated fatty acids are fatty acids that contain more than one double bond in their backbone .this class includes many important compounds ,such as essential fatty acids and those that give drying oils their characteristic property .
Polyunsaturated fatty acids can be classified in various groups by their chemical structures :
1) methylene-interrupted polyenes
2) conjugated fatty acids
based on the length of their carbon backbone ,they are sometimes classified in two groups :
1) short chain polyunsaturated fatty acids : with 18 carbon atoms .
2) long - chain polyunsaturated fatty acids : with 20 or more carbon atoms .
Learn more about fatty acids here :
brainly.com/question/26353151
#SPJ4
please I need help ASAP
Lead nitrate decomposes on heating as indicated in Equation. 2Pb(NO3)2(s) 2PbO(s) + 4NO₂(g) + O₂(g) (4.8) If a volume of 112 cm³ of oxygen gas was collected at STP when a sample of lead nitrate was completely decomposed by heating, calculate the; (a) mass of the lead nitrate sample. (b) mass of lead(II) oxide produced. (c) Volume of nitrogen dioxide gas produced at STP. (Pb=207, N = 14, O=16; molar volume of gas at STP = 22.4 dm³)
Answers
Answer:
To solve this problem, we'll need to use stoichiometry and the molar ratios from the balanced chemical equation. Here's how you can calculate the values:
(a) Mass of the lead nitrate sample:
From the balanced equation, we can see that 2 moles of lead nitrate (Pb(NO3)2) produce 1 mole of oxygen gas (O2). We know that the volume of oxygen gas collected is 112 cm³, which is equal to 112/1000 = 0.112 dm³ (converting cm³ to dm³).
According to the molar volume of gas at STP (22.4 dm³), 1 mole of any gas occupies 22.4 dm³ at STP. Therefore, the number of moles of oxygen gas can be calculated as:
moles of O2 = volume of O2 / molar volume at STP
moles of O2 = 0.112 dm³ / 22.4 dm³/mol = 0.005 mol
Since 2 moles of lead nitrate produce 1 mole of oxygen gas, we can determine the number of moles of lead nitrate as:
moles of Pb(NO3)2 = 2 * moles of O2
moles of Pb(NO3)2 = 2 * 0.005 mol = 0.01 mol
To calculate the mass of the lead nitrate sample, we'll use its molar mass:
mass of Pb(NO3)2 = moles of Pb(NO3)2 * molar mass of Pb(NO3)2
mass of Pb(NO3)2 = 0.01 mol * (207 g/mol + 2 * 14 g/mol + 6 * 16 g/mol)
mass of Pb(NO3)2 = 0.01 mol * 331 g/mol
mass of Pb(NO3)2 = 3.31 g
Therefore, the mass of the lead nitrate sample is 3.31 grams.
(b) Mass of lead(II) oxide produced:
From the balanced equation, we can see that 2 moles of lead nitrate (Pb(NO3)2) produce 2 moles of lead(II) oxide (PbO). So, the number of moles of PbO produced is equal to the number of moles of Pb(NO3)2.
mass of PbO = moles of PbO * molar mass of PbO
mass of PbO = 0.01 mol * (207 g/mol + 16 g/mol)
mass of PbO = 0.01 mol * 223 g/mol
mass of PbO = 2.23 g
Therefore, the mass of lead(II) oxide produced is 2.23 grams.
(c) Volume of nitrogen dioxide gas produced at STP:
From the balanced equation, we can see that 2 moles of lead nitrate (Pb(NO3)2) produce 4 moles of nitrogen dioxide gas (NO2). So, the number of moles of NO2 produced is twice the number of moles of Pb(NO3)2.
moles of NO2 = 2 * moles of Pb(NO3)2
moles of NO2 = 2 * 0.01 mol = 0.02 mol
Using the molar volume of gas at STP, we can calculate the volume of nitrogen dioxide gas:
volume of NO2 = moles of NO2 * molar volume at STP
volume of NO2 = 0.02 mol * 22.4 dm³/mol = 0.448 dm³
Therefore, the volume of nitrogen dioxide gas
The komodo dragon prefers to hunt by stealth, but can briefly run 6.5 miles for half an hour. What is the average speed in miles per hour?
Answers
The average speed in miles per hour, given the data is 13 miles per hour
What is speed?Speed is the distance travelled per unit. Mathematically, it can be expressed as:
Speed = distance / time
What is average speed?This is the total distance travelled divided by the total time taken to cover the distance.
Average speed = total distance / total time
How to determine the average speedThe following data were obtained from the question:
Total distance = 6.5 mileTotal time = 1/2 hour = 0.5 hourAverage speed =?The average speed can be obtained as follow:
Average speed = total distance / total time
Average speed = 6.5 / 0.5
Average speed = 13 miles per hour
Thus, the average speed is 13 miles per hour
Learn more about speed:
https://brainly.com/question/680492
#SPJ1
The voltage produced by the colorimeter is __________ to the absorbance of the sample and ____________ to the light intensity.
A) Directly proportional, indirectly proportional
B) Directly proportional, directly proportional
C) Indirectly proportional, indirectly proportional
D) Indirectly proportional, directly proportional
E) None of the above
Answers
The voltage produced by the colorimeter is __________ to the absorbance of the sample and ____________ to the light intensity.
E) None of the above
Relation between transmittance and absorbance is as follows.
Therefore we know that the amount of light that passes through a solution is known as transmittance.
It can be expressed as follows;
T= \(\frac{I_{t} }{I_{0} }\)
Now we know that, I, is the intensity of transmitted light.
I is the initial intensity of light beam.
Then the colorimeter produces an output voltage which is linearly varies with transmittance that is light intensity.
Relation between absorbance and transmittance is as follows:
A = log (\(\frac{1}{T}\))
The reciprocal of transmittance of the sample varies logarithmically varies with absorption.
Therefore, we can say that the voltage produced by the colorimeter is varies logarithmically reciprocal to the absorbance of the sample and linear to the light intensity.
To learn more about light intensity, click here:
brainly.com/question/9195922
#SPJ4
Calculate the concentration of flavonoids in apples grown with reflective ground cover relative to the concentration of flavonoids in apples grown without reflective ground cover.
Answers
In a given scenario, apples grown with reflective ground cover have a 25% higher flavonoid concentration compared to those grown without it.
The concentration of flavonoids in apples grown with reflective ground cover can be compared to the concentration in apples grown without it to understand the impact of this agricultural method on fruit quality. Flavonoids are a group of plant compounds known for their antioxidant properties, and higher concentrations are often associated with greater health benefits.
In order to calculate the concentration of flavonoids in both types of apples, you would need to gather samples from each group and perform a quantitative analysis, such as high-performance liquid chromatography (HPLC). This would allow you to accurately determine the flavonoid content in each sample.
After analyzing the data, you would calculate the average concentration of flavonoids for apples grown with reflective ground cover and those grown without it. To compare these values, you could calculate the relative difference between the two averages, which can be expressed as a percentage.
For example, if apples grown with reflective ground cover had an average flavonoid concentration of 50 mg/kg, and those grown without it had an average of 40 mg/kg, you would find the relative difference as follows:
(50 - 40) / 40 = 0.25 or 25%
In this hypothetical scenario, apples grown with reflective ground cover have a 25% higher flavonoid concentration compared to those grown without it. Keep in mind that actual results may vary and are dependent on factors such as cultivar, growing conditions, and sample size.
To know more about flavonoids, refer to the link below:
https://brainly.com/question/30866030#
#SPJ11
Consider the reaction that will take place between (S)-2-bromobutane and sodium azide in DMF (Dimethylformamide). What is the product of this reaction (give proper stereochemistry of the product formed)? What is the mechanism of this reaction? Draw the structure of the transition state of this reaction.
Answers
When (S)-2-bromobutane is reacted with sodium azide in Dimethylformamide (DMF), the product formed is (S)-2-azidobutane. Here, the stereochemistry of the product formed is retained in the (S)-configuration. Therefore, (S)-2-azidobutane is obtained as the product formed.
What is the mechanism of this reaction?The mechanism of the reaction is a nucleophilic substitution reaction, commonly known as SN2 reaction. The steps involved in the reaction mechanism of (S)-2-bromobutane with sodium azide in DMF is as follows:
1) Initially, DMF works as the solvent, which solvates sodium azide and makes it more nucleophilic.
2) The lone pair of electrons of the nitrogen atom in sodium azide attacks the carbon atom of the chiral center of the (S)-2-bromobutane.
3) This attack causes the carbon-bromine bond to break and releases the bromine as bromide ion.
4) The resulting intermediate formed is a carbonium ion, in which the nucleophile attaches to the carbon atom that is bonded to the leaving group, leading to inversion of stereochemistry.
5) Finally, the product obtained is (S)-2-azidobutane.
learn more about nucleophilic substitution here
https://brainly.com/question/29382322
#SPJ11
Mauren and Esteban have been investigating various reactions to find out if they are exothermic or endothermic
Which of these reactions are endothermic and which are exothermic
Answers
Having problems figuring out molar mass in general. steps would be most appreciated.
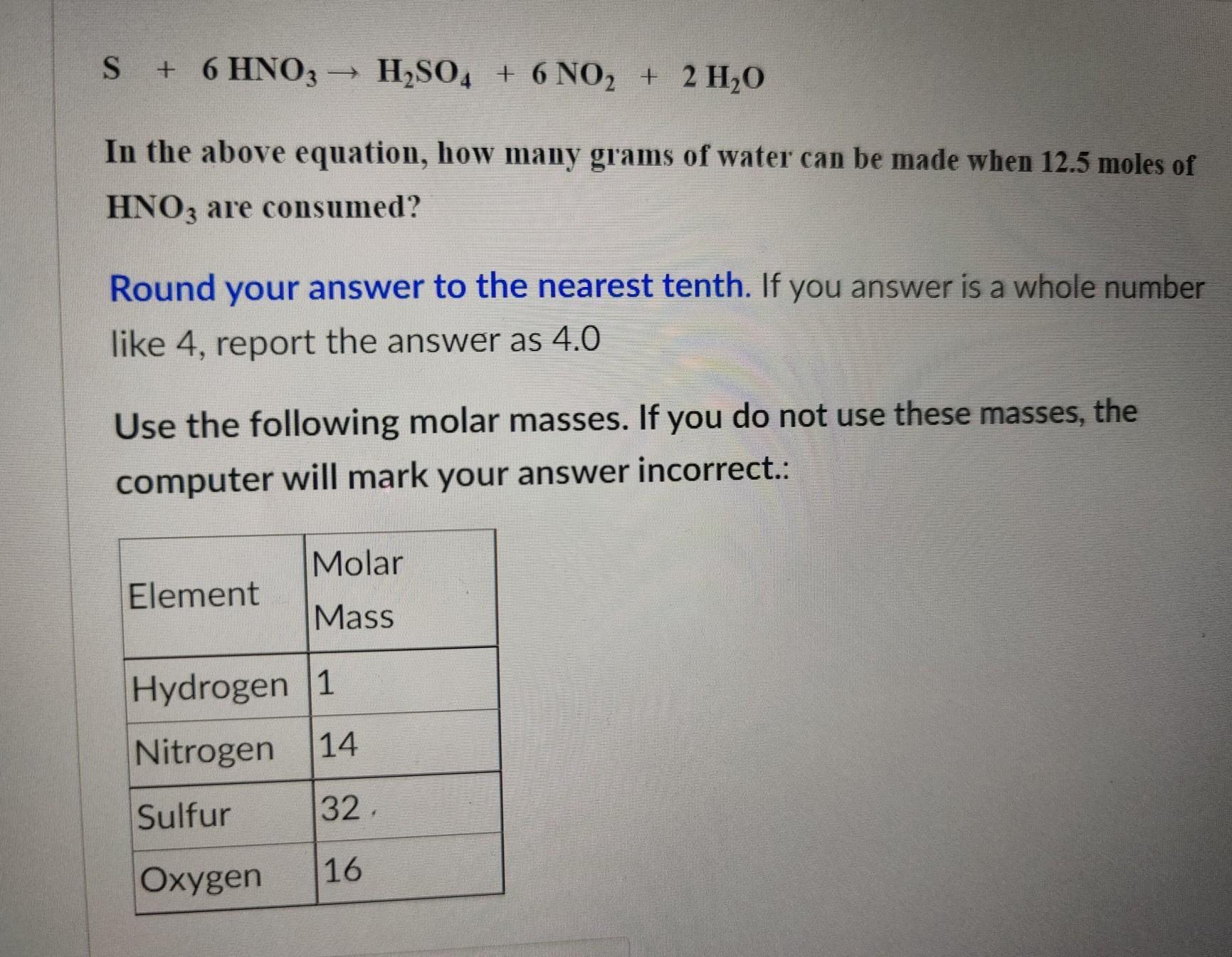
Answers
Answer:
The periodic table
Explanation:
As you can see in the screenshot. Each element on the periodic table has an atomic number and a mass number. The atomic numbers are arranged in numerical order from right to left in the periodic table. The second number there is the atomic mass.
When doing stoichiometric ratios, you need to use the atomic mass of each element to determine the molecular/molar mass.
example. Find the molar mass of
H₂SO₄
Hydrogen (2) + sulphur (1) +oxygen (4)
= atomic mass of H(2)+ atomic mass S(1)+ atomic mass oxygen(4)
=1(2) + 32(1)+16(4)
=2+32+64
=98
N.B We do not use the co-efficient when looking for molar mass, we only use the atom and subscript
which statement best explains what would happen if a reactant were added to a system in equilibrium?(1 point) responses the system would shift toward the products to enhance the change. the system would shift toward the products to enhance the change. the system would shift toward the reactants to oppose the change. the system would shift toward the reactants to oppose the change. the system would shift toward the products to oppose the change. the system would shift toward the products to oppose the change. the system would shift toward the reactants to enhance the change. the system would shift toward the reactants to enhance the change.
Answers
In a system at equilibrium, the forward and reverse reactions are occurring at equal rates. This means that the concentration of reactants and products is stable and no net change is observed. However, if a reactant is added to the system, the equilibrium is disrupted and the system is no longer at equilibrium.
The Le Chatelier's Principle states that when a system at equilibrium is disturbed, the system will shift in a way that opposes the change. In the case of adding a reactant, the system will shift towards the products in order to consume the added reactant and restore equilibrium. This is because the increase in reactant concentration is seen as a stress on the system and the system will respond by reducing that stress.
Conversely, if a product is added to the system, the system will shift towards the reactants to consume the added product and restore equilibrium. The system will always try to minimize the effect of the disturbance on the equilibrium.
It is important to note that the extent of the shift in equilibrium will depend on the relative concentrations of the reactants and products, as well as the equilibrium constant of the reaction. The system will shift in a way that minimizes the disturbance while still maintaining the equilibrium constant.
In conclusion, when a reactant is added to a system at equilibrium, the system will shift towards the products to oppose the change and restore equilibrium. The same principle applies when a product is added, with the system shifting towards the reactants.
for more such questions on equilibrium
https://brainly.com/question/19340344
#SPJ11
calculate the avergae kineti c energy of the ch4 molecules in a sample of methane gas at 273k and 546k
Answers
The average kinetic energy of CH4 molecules in a sample of methane gas at 273K and 546K
6.00 x 10^-21 J and 1.19 x 10^-20 J
To calculate the average kinetic energy of CH4 molecules in a sample of methane gas at 273K and 546K, we need to use the formula:
KEavg = (3/2) kT
where KEavg is the average kinetic energy, k is the Boltzmann constant (1.38 x 10^-23 J/K), and T is the temperature in Kelvin.
At 273K, the average kinetic energy of CH4 molecules is:
KEavg = (3/2) x (1.38 x 10^-23 J/K) x (273K)
At 546K, the average kinetic energy of CH4 molecules is:
KEavg = (3/2) x (1.38 x 10^-23 J/K) x (546K)
Therefore, the average kinetic energy of CH4 molecules in a sample of methane gas increases as the temperature increases. This is because at higher temperatures, the molecules have more kinetic energy and move faster.
To learn more about : kinetic
https://brainly.com/question/25959744
#SPJ11
Describe the difference between the rate of diffusion seen for sodium and urea.
Answers
1) If you have 2.6 moles of iron (III) oxide, how many molecules of iron (III)
oxide do you have?
Answers
Answer:
234
Explanation:
so 3 x 3 x 26 =234
which two events are apart of the rock cycle
Answers
Answer:
The key processes of the rock cycle are crystallization, erosion and sedimentation, and metamorphism.
Explanation:
mark me brainliest!!

Answer:
Earths heat and hydrological cycle
Explanation:
even cold air radiates what?
Answers
Answer:
Cold air also radiates Heat.
Heat transfer is awesome!!
Even cold air radiates electromagnetic waves, specifically in the form of thermal radiation.
Thermal radiation is the emission of electromagnetic waves by any object that has a temperature above absolute zero. All objects, including cold air, possess thermal energy due to the motion of their molecules.
According to the laws of thermodynamics, objects at any temperature above absolute zero emit thermal radiation.
The intensity and frequency distribution of the radiation depends on the object's temperature and its emissivity, which is a measure of how efficiently it emits radiation.
To learn more about electromagnetic waves, follow the link:
https://brainly.com/question/29774932
#SPJ6
NEED ANSWER IMMEDIATELY: 14. Problem: Uranium-238 has a mass number of 238 with 146 neutrons in the
nucleus. An isotope, Uranium-235 has 143 neutrons in the nucleus. What is
the atomic number of Uranium?
Answers
Answer:
92
Explanation:
What is carbon? What does it do?