a mass of 48.5 g of a certain substance can be dissolved in 29.1 ml of water at 20 degrees c calculate solubility of this substance per gram of water
Answers
A mass of 48.5 g of a certain substance can be dissolved in 29.1 ml of water at 20° C, the solubility of this substance is 1.67 g.
We know that, Density of water = 1 g / mL
= 29.1 mL x 1 g
= 29.1 g water
Solubility = (Mass of solute / Mass of solvent) × 100
= ( 48.5 g / 29.1 g ) × 100
= 167 g
Here, Solubility of substance is 167 g per 100 gm of water
So, 167 × 1 / 100 = 1.67 g of the substance can be dissolved per gram of water.
Thus, 1.67 g of the substance can be dissolved per gram of water.
To learn more about Solubility, Here :
https://brainly.com/question/10915599?referrer=searchResults
#SPJ4
Related Questions
What are two types of matter that are considered pure?.
Answers
Answer: Elements and compounds are both examples of pure substances.
Explanation:
6) Aluminum bromide decomposes
Answers
Answer:
The reaction begins and builds up heat. This heat causes the aluminum to melt and float on top of the liquid bromine (which is producing a cloud of vapor because the heat is also boiling it). Wherever the two elements meet, sparks, heat, and light are given off.
Explanation:
be more specific
Predict what must happen when a beaker containing a solution of lithium chloride is heated. (choose all that apply) a. The reaction will become endothermic. b. The delta(H) of solution value will decrease. c. The solubility of lithium chloride will increase. d. Energy will be transferred into the system.
Answers
Answer:
c. The solubility of lithium chloride will increase
How are some islands formed? Do you think islands can form at any time? Where are we more likely to find islands?
Answers
Answer:
Islands are formed by volcanic eruptions and tectonic plates movements.
Explanation:
Islands are land masses that are covered with water on all its sides. Islands are mainly formed by the volcanic eruptions or by the tectonic forces. When volcanoes erupt, they try to build up layers of the lava that eventually emerged through the water surface and forms an island.
Islands can be also formed when tectonic plates forces each other beneath the water body and land masses rise up to form islands.
Yes islands can be formed at any time. But it takes time for the formation of an island.
Islands are more likely to occur on the seas, oceans, lakes or river, etc.
Which of the following samples of baking soda wold react the fastest with
vinegar? *
1 point
powder
they all react at the same rate
O small crystal
small cube
Answers
Write the chemical equation that the diagram
represents.

Answers
In the given diagram, the first atom contains one valence electron and the second have 7 valence electrons. The first atom donates the one electron to the second atom and both achieve octet and forms and ionic compound.
What are ionic compounds?Ionic compounds are formed between a metal and non-metal. Metals are rich in electrons and easily loss one or more electrons to other atoms. Non-metals are electron deficient and can gain electron from metals. These electron loss and gain between atoms forms the ionic bond between them.
In the diagram the first atom contains total 11 electron of which one is valence electron. By donating this extra electron the atom can achieve octet. The second one contains total 17 electrons of which 7 are valence electrons, thus need one more electron to attain octet.
The first atom represents sodium atom and the second one is chlorine atom. Sodium donates its valence electron to Cl and form the ionic compound NaCl.
Na⁺ + Cl⁻ ⇒ NaCl
Find more on ionic compounds:
https://brainly.com/question/9167977
#SPJ1
How many moles are in 98.3 grams of NaF?
Answers
Answer:
1 mole is equal to 6.022x10²³
Answer:
2.979 mol
Explanation:
mass of NaF=14+19
=33g
33g of NaF is the mass of 1 mol
98.3g of NaF is the mass of (98.3/33)*1=2.979 mol
A swimming pool reading reports that chlorine is present at 130 ppm. How many grams of chlorine are present per liter of pool water?
Answers
130 ppm will contain 0.12985167 grams of chlorine present in per liter of pool water.
What is ppm?This is an abbreviation for "parts per million" and it also can be expressed as milligrams per liter (mg/L).
1001.142303 ppm is equal to 1 g/L
So, 130 ppm will contain \(\frac{130}{1001.142303}\) grams of chlorine present in per liter of pool water which is equal to 0.12985167 gram/L.
Learn more about ppm here:
https://brainly.com/question/2618749
#SPJ1
What is a good description of bacterial reproduction? (1 point)
Responses
rapid reproduction through binary fission
slow reproduction through sexual reproduction
fast reproduction through sexual reproduction
slow reproduction through binary fission
Answers
A good description of bacterial reproduction is rapid reproduction through binary fission. That is option A.
What is binary fission?Binary fission is defined as the type of asexual reproduction whereby an organism multiplies to form new organisms through the separation of the body into two new bodies.
The process of binary fission include the following:
The organism such as bacteria, duplicates its genetic material( deoxyribonucleic acid).It then divides into two parts (cytokinesis),After each division, the new organism receive one copy of DNA. each.Under ideal conditions some bacterial species may divide every 10–15 minutes—a doubling of the population at these time intervals.
Therefore, the good description of bacterial reproduction is rapid reproduction through binary fission.
Learn more about reproduction here:
https://brainly.com/question/29762377
#SPJ1
How does a scientist make two solutions with the same molarity?
OA. By dissolving the maximum amount of each substance in the
same volume of water
OB. By dissolving the same number of moles of each substance in the
same volume of water
OC. By dissolving 1 mole of each substance in enough water to make
sure dissolving is complete
OD. By dissolving the same number of grams of each substance in the
same volume of water
SUBMIT
Answers
The term molarity is an important method which is used to calculate the concentration of a solution. By dissolving the same number of moles of each substance in the same volume of water we can make solutions of same molarity. The correct option is B.
Molarity is defined as the number of moles of the solute present per litre of the solution. It is represented as 'M' and its unit is mol / L. The term molarity is also called the molar concentration.
When same number of moles of substances are dissolved in the same volume of water, then the two solutions have same molarity.
Thus the correct option is B.
To know more about Molarity, visit;
https://brainly.ph/question/1634511
#SPJ1
part A
Cd(s)+Sn2+(aq)→Cd2+(aq)+Sn(s)
Write the anode half-reaction.
Express your answer as a ionic equation. Identify all of the phases in your answer.
Part B
Cd(s)+Sn2+(aq)→Cd2+(aq)+Sn(s)
Write the cathode half-reaction.
Express your answer as a ionic equation. Identify all of the phases in your answer.
Part C
2Al(s)+3Cd2+(aq)→2Al3+(aq)+3Cd(s)
Write the anode half-reaction.
Express your answer as a ionic equation. Identify all of the phases in your answer.
Part D
2Al(s)+3Cd2+(aq)→2Al3+(aq)+3Cd(s)
Write the cathode half-reaction.
Express your answer as a ionic equation. Identify all of the phases in your answer.
Answers
Part A:
a. Anode half-reaction: Cd(s) → Cd₂+(aq) + 2e⁻
b. Ionic equation: Cd(s) → Cd₂+(aq) + 2e⁻
c. Phases: solid (s), aqueous (aq)
Part B:
a. Cathode half-reaction: Sn₂⁺(aq) + 2e⁻ → Sn(s)
b. Ionic equation: Sn₂⁺(aq) + 2e⁻ → Sn(s)
c. Phases: aqueous (aq), solid (s)
Part C:
a. Anode half-reaction: Al(s) → Al₃⁺(aq) + 3e⁻
b. Ionic equation: Al(s) → Al₃⁺(aq) + 3e⁻
c. Phases: solid (s), aqueous (aq)
Part D:
a. Cathode half-reaction: 3Cd₂⁺(aq) + 6e⁻ → 3Cd(s)
b. Ionic equation: 3Cd₂⁺(aq) + 6e⁻ → 3Cd(s)
c. Phases: aqueous (aq), solid (s)
Part A: The anode half-reaction for the equation Cd(s) + Sn₂⁺(aq) → Cd₂+(aq) + Sn(s) is the oxidation of cadmium. Part B: The cathode half-reaction for the equation Cd(s) + Sn₂⁺(aq) → Cd₂⁺(aq) + Sn(s) is the reduction of tin. Part C: The anode half-reaction for the equation 2Al(s) + 3Cd₂⁺(aq) → 2Al₃+(aq) + 3Cd(s) is the oxidation of aluminum. Part D: The cathode direction for the equation 2Al(s) + 3Cd₂⁺(aq) → 2Al₃⁺(aq) + 3Cd(s) is the reduction of cadmium.
Learn more about anode half-reaction: https://brainly.com/question/27982574
#SPJ11
a cell biologist measures the width of a cell. The width is 76um. what is the width in meters? write in scientific notation.
Answers
The width of the cell, which is 76 micrometers will be have a scientific notation value of 7.6 × 10-⁵metres
CONVERSION FACTOR:
According to this question, a cell biologist measures the width of a cell and found out it is 76um. 76um means 76 micrometersMicrometers (um) is a smaller unit of measurement than the metres. The conversion factor of metres and micrometers is as follows:1um = 10-⁶m
Hence, 76um will be equal to 76 × 10-⁶m= 7.6 × 10-⁵mTherefore, the width of the cell in metres is 7.6 × 10-⁵m.
Learn more: https://brainly.com/question/17654754?referrer=searchResults
INACCURATE STATEMENT: At the time of the big bang, all the matter and energy in the universe was in a tiny corner of space. Since then, it has expanded to fill up the whole universe.
Choose why this statement is inaccurate using the EVIDENCE that refutes it (proves it wrong).
1 EVIDENCE: Scientists believe the temperature of the universe immediately after the big bang was 100 billion *C. Today, the temperature of the universe is -275*C.
2 EVIDENCE: Scientists believe the very first galaxies began forming about 1 billion years after the big bang.
3 EVIDENCE: Blue light has shorter wavelengths than red light.
4 EVIDENCE: Scientists have observed galaxies are moving away from us.
5 EVIDENCE: The big bang marks the beginning of space and time.
choose only one
Answers
The evidence that refutes the statement is: 4 EVIDENCE: Scientists have observed galaxies are moving away from us.
According to the observations made by astronomers, galaxies in the universe are not only moving away from each other, but they are also moving away from us.
This phenomenon is known as the expansion of the universe, and it contradicts the idea that all matter and energy in the universe was initially concentrated in a tiny corner of space during the time of the big bang and has since filled up the entire universe.
The observation that galaxies are moving away from us suggests that the universe is expanding in all directions. This expansion implies that the universe was not initially confined to a specific location but rather underwent a rapid expansion from a highly dense and hot state.
Therefore, the idea that all matter and energy in the universe was initially concentrated in a small corner of space and then expanded to fill up the whole universe is inaccurate based on the evidence of the observed expansion of galaxies. Evidence 4
For more such questions on galaxies visit:
https://brainly.com/question/29357062
#SPJ8
write the structural (not molecular!) formulas for the products of the following three reactions. name (just name!) the reaction mechanism.
Answers
Structural Formula of Ethene: H2C=CH2
Structural Formula of Ethane: CH3-CH3
This reaction is known as hydrogenation.
The question asks for the structural formulas of the products and the name of the reaction mechanism for three given reactions. However, the specific reactions and their corresponding mechanisms are not provided.
To provide a helpful response, I'll give you an example of a reaction, its products, and the name of the mechanism.
Example:
Reaction: The reaction of an alkene with hydrogen gas (H2) in the presence of a catalyst (e.g., platinum) to form an alkane.
Products: In this reaction, the alkene reacts with hydrogen to form an alkane. For example, if the alkene is ethene (C2H4), the product would be ethane (C2H6).
Structural Formula of Ethene: H2C=CH2
Structural Formula of Ethane: CH3-CH3
Reaction Mechanism: This reaction is known as hydrogenation. Hydrogenation is a type of addition reaction, where hydrogen atoms are added across the carbon-carbon double bond of the alkene to form a single bond and convert it into an alkane.
Please note that without specific reactions provided, I can only give you an example to illustrate the process of determining the products and reaction mechanism. If you have specific reactions in mind, please provide them, and I will be glad to assist you further.
learn more about hydrogenation on:
https://brainly.com/question/13468771
#SPJ11
what size volumetric flask would be useful in diluting 100 ml of a 3.0 m solution of sucrose to make a 0.60 m solution. dilutions worksheet answers
Answers
The size of volumetric flask that would be useful in diluting 100 ml of a 3.0 M solution of sucrose to make a 0.60M solution is 500mL.
How to calculate volume?The volume of a solution can be calculated by using the following expression;
C1V1 = C2V2
Where;
C1 = initial concentrationC2 = final concentrationV1 = initial volumeV2 = final volume3 × 100 = V2 × 0.60
300 = 0.6V2
V2 = 300 ÷ 0.6
V2 = 500mL
Therefore, the size of volumetric flask that would be useful in diluting 100 ml of a 3.0 M solution of sucrose to make a 0.60M solution is 500mL.
Learn more about volume at: https://brainly.com/question/12127540
which of the following would typically have the highest concentration of alcohol?
Answers
The drink that would typically have the highest concentration of alcohol is hard liquor. Hard liquors are made through the process of distillation, which increases the alcohol content to a higher level.
Hard liquor is defined as liquor with an alcohol content of 30% or more, or 60 proof or higher. This means that they have a higher concentration of alcohol than beer, wine, or other drinks.Liquors are classified into different categories based on their alcohol content, which determines the intensity of their flavors and aromas. Some of the most popular types of hard liquor include whiskey, vodka, rum, gin, tequila, brandy, and cognac.
These liquors are usually consumed in small quantities, and they are often mixed with other ingredients to create cocktails. Their high alcohol content makes them perfect for people who want to drink alcohol without consuming large amounts of liquid.The alcohol content of hard liquor varies depending on the type of liquor and the brand. However, the average alcohol content for most hard liquors is between 35% and 45%. Some of the strongest hard liquors can have an alcohol content of up to 90%. Therefore, it is important to consume hard liquor in moderation to avoid harmful effects on the body.
To know more about concentration visit;-
https://brainly.com/question/3045247
#SPJ11
can the atomic mass of an element vary? can the atomic mass of an element vary? no, it is fixed; otherwise a new element will be formed. yes. adding or losing neutrons will change the atomic mass without forming a different element. yes. adding or losing protons will change the atomic mass without forming a different element. yes. adding or losing electrons will substantially change the atomic mass.
Answers
Answer:Yes. Adding or losing neutrons will change the atomic mass without forming a different element.
Explanation: Brainly
What kind of mixture is a solution? Name the different constituents of a solution
Answers
Answer:
homogeneous mixture
Explanation:
two or more substances
a component that dissolves and a solvent
manganese is a transition metal. consider the isotope: mn-59. how many protons are in an atom of mn-59 if the atom has a charge of 5?
Answers
The atomic number of an element represents the number of protons in an atom of that element. Since the isotope given is Mn-59, the atomic number of manganese (Mn) remains the same, which is 25.
If an atom of Mn-59 has a charge of +5, it means that it has lost 5 electrons. The number of protons in an atom is equal to its atomic number, and the number of electrons is equal to the number of protons in a neutral atom. Therefore, if the atom has lost 5 electrons, the number of protons remains the same, which is 25.
So, an atom of Mn-59 with a charge of +5 has 25 protons.
learn more about protons here
https://brainly.com/question/12535409
#SPJ11
the addition of ammonia and ________ to water produces a buffer solution. a) NH4Cl
b) NaBr
c) NH3
d) NaNO3
e) Kl
Answers
Answer:
The addition of ammonia (NH3) and NH4Cl to watwr produces a buffer solution.
Explanation:
Because, to make a buffer solution from a weak base compound, a salt of the conjugate acid can be added.
Because NH3 has a conjugate acid, namely NH4, the suitable salt added for NH3 to become a buffer solution is NH4Cl.
Check Your Learning
What mass of CO is required to react with 25.13 g of Fe2O3
according to the equation Fe₂O3 + 3 CO2 Fe + 3CO₂?
Answers
Answer:
convert the 25.13 g of Fe2O3 into moles by dividing by its molar mass (159.69) to get that. When looking at the stoichiometric coefficients, we can see that for every 1 mole of Fe2O3, there needs to be 3 moles of CO.
Explanation:
What is NOT a sign of chemical change?
A. New Color
B. New gas
C. New temperature
D. New state of matter
Answers
Answer:
d
Explanation:
Hey guys I'm under a lot of pressure to finish my Chemistry assignment before the term ends, and help will be appreciated even if you answer just one.
1. What is the pressure on a gas at 350. K if the pressure is 2.62 atm at 300. K?
2. The volume of a gas at 298K is 0.346 L when the pressure is 201 kPa, what will the volume be when the pressure is 152 kPa at 298K?
3. In a rigid, 6 L container, the pressure of N2 gas is 0.592 atm at 305 K, what temperature will the gas be if the pressure is 0.891 atm?
4. The pressure of a sample of CO2 was 2.00 atm when the temperature was 30.45 ℃, what will the pressure be if the temperature of the sample is heated to 92.01℃?
Answers
Explanation:
Hello
I answered all your questions And I explained it to you in the photo sent.
Good luck

The average rate of a reaction is the rate of reaction at any given time.
A) True
B) False
Answers
B) False. The average rate of a reaction is the change in the concentration of a reactant or product over a certain time interval, usually calculated by dividing the change in concentration by the time interval.
It is not the rate of reaction at any given time, but rather an average of the rate of reaction over a certain period of time.
The rate of reaction at any given time is called the instantaneous rate of reaction, and it is calculated by finding the slope of the tangent line to the concentration-time curve at a particular point in time. The instantaneous rate of reaction can change over time as the concentration of reactants and products change, whereas the average rate of reaction remains constant over the time interval for which it is calculated.
Suppose a reaction occurs according to the equation A → B. The rate of this reaction can be expressed as:
Rate = - d[A]/dt = d[B]/dt
where d[A]/dt is the rate of disappearance of A and d[B]/dt is the rate of appearance of B. The negative sign in the equation indicates that the rate of disappearance of A is equal in magnitude but opposite in sign to the rate of appearance of B.
The instantaneous rate of the reaction at a particular time t can be calculated by finding the slope of the tangent line to the concentration-time curve of either A or B at that time. This tangent line represents the rate of reaction at that specific moment in time.
On the other hand, the average rate of the reaction over a certain time interval (t1 to t2) can be calculated by taking the difference in the concentration of A or B at time t2 and time t1, and dividing it by the time interval (t2 - t1):
Average rate = (Δ[A]/Δt)avg = - (Δ[B]/Δt)avg
where (Δ[A]/Δt)avg is the average rate of disappearance of A and (Δ[B]/Δt)avg is the average rate of appearance of B over the time interval.
Therefore, the average rate of a reaction is not the rate of reaction at any given time, but rather an average of the rate of reaction over a certain period of time. The instantaneous rate of reaction, on the other hand, is the rate of reaction at a specific moment in time.
To know more about time interval
brainly.com/question/14512776
#SPJ11
Acetone (nail polish remover) has a density of 0.7857 g >cm3.
a. What is the mass in g of 28.56 mL of acetone?
b. What is the volume in mL of 6.54 g of acetone?
Answers
If acetone has a density of 0.7857 \(\frac{g}{cm^{3} }\) the mass in grams of point A is 22.4 g and the volume at point B is 8.32 mL.
What is acetone?Acetone is known as a chemical substance that is usually found in the environment but can also be produced artificially. Acetone is a polar organic product that interacts very well with water molecules, generating dipole-dipole relationships.It is colorless with a distinctive smell and taste, we find it in products known as cleaning and personal care products, but we can also use it as a solvent for substances.
Also in the environment in plants, trees and in volcano emissions or in forest fires, it does not become toxic in low doses but if it is exposed to an individual in high doses it can become fatal.
In the statement we can find that acetone has a density of 0.7857 \(\frac{g}{cm^{3} }\).
Therefore, we can confirm that if acetone has a density of 0.7857 \(\frac{g}{cm^{3} }\) the mass in grams of point A is 22.4 g and the volume at point B is 8.32 mL.
To learn more about acetone visit: https://brainly.com/question/13334667?referrer=searchResults
#SPJ1
Explain why ethanamide is solid at room temperature but 1-aminobutane liquid even when the have the same molecular mass
Answers
Even though ethanamide and 1-aminobutane have the same molecular mass, they have different intermolecular forces due to their polarity, resulting in different physical states at room temperature.
Ethanamide (\(CH_3CONH_2\)) is a polar solid at room temperature due to its strong intermolecular hydrogen bonds, which create a lattice structure that gives it solid-state rigidity. Ethanamide, also known as acetamide, is a polar molecule with a carbonyl group that can form hydrogen bonds with other molecules like it. The hydrogen bonding interaction between the molecules is what keeps the solid-state of ethanamide intact.
In contrast, 1-aminobutane (\(C_4H_{11}N\)) is a nonpolar liquid at room temperature, despite having the same molecular mass as ethanamide. It has a linear structure and is an amine, which means it contains a nitrogen atom. However, 1-aminobutane is nonpolar, making it less likely to form intermolecular hydrogen bonds like those in ethanamide.
The fact that 1-aminobutane is nonpolar means that it lacks the strong intermolecular forces, so it exists as a liquid at room temperature and pressure. The molecules can pack more closely together due to the strong intermolecular forces, resulting in a more rigid lattice structure in the solid-state.
For more such questions on ethanamide visit;
https://brainly.com/question/15315152
#SPJ8
If some water splashed out of your coffee cup when transferring the metal washers into it, how would this affect the final specific heat capacity of the metal
Answers
If some water splashed out of the coffee cup during the transfer of metal washers, it would lead to a decrease in the mass of water in the coffee cup. This would result in a decrease in the total heat capacity of the system, which would affect the final specific heat capacity of the metal.
Specific heat capacity is defined as the amount of heat energy required to raise the temperature of one unit mass of a substance by one degree Celsius. Thus, a decrease in the mass of water in the system would affect the calculation of the heat energy transferred to the metal, which would affect the specific heat capacity calculation of the metal.
Therefore, it is important to ensure that all the water and metal are transferred accurately and completely to obtain precise and accurate results for the specific heat capacity of the metal.
To know more about the metal washers refer here :
https://brainly.com/question/14258858#
#SPJ11
4. The following element contains 12 protons and a mass number of 24. Which of the following statements is NOT correct about it? * O It contains 24 neutrons O It contains 12 electrons O Its atomic number is 12 O its contains 24 nucleons.
Answers
Answer:
It contains 24 neutrons.
Explanation:
Mass number = Protons + Neutrons
If you were to have 24 neutrons, the mass number would be 36, invalidating the known information. You have 12 protons and 12 neutrons to form a mass number of 24. Remember that your protons are the same thing as the atomic number, so you can make an equation using your known information to derive true statements.
What conditions make G always positive?
A. G is always positive when enthalpy and entropy both decrease.
B. G is always positive when enthalpy increases and entropy
decreases.
C. G is always positive when enthalpy decreases and entropy
increases.
D. G is always positive when enthalpy and entropy both increase.
Answers
Answer:
b
Explanation:
G is always positive when enthalpy increases and entropy decreases.
What determines if a reaction is spontaneous?The temperature can be the deciding factor in spontaneity when the enthalpy and entropy terms have opposite signs: If ΔH is negative, and –TΔS positive, the reaction will be spontaneous at low temperatures (decreasing the magnitude of the entropy term).
Why is negative Gibbs free energy spontaneous?Where ΔH is the enthalpy change (the heat of the reaction) and ΔS is the change in entropy. If the free energy is negative, we are looking at changes in enthalpy and entropy that favor the process and it occurs spontaneously.
Learn more about the spontaneous reaction here https://brainly.com/question/24376583
#SPJ2
PLS HELP ME After completing the Gold Foil Experiment, Ernest Rutherford proposed that the positive charge of an atom was concentrated in a dense central core or
nucleus. What evidence from the experiment did he use to support this idea? Your answer should reference the image.
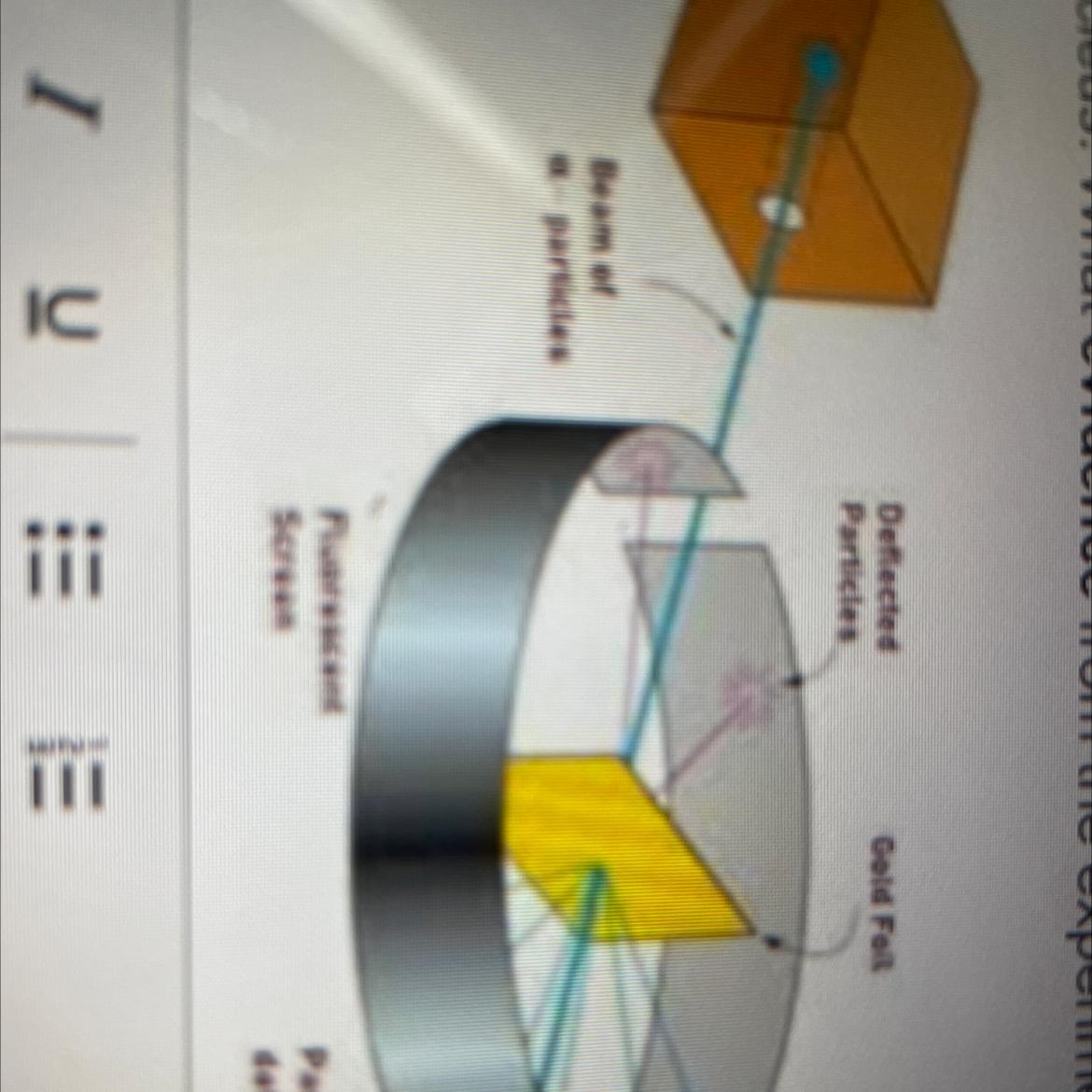
Answers
Answer:
A piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. This showed that the gold atoms were mostly empty space. Some particles had their paths bent at large angles. A few even bounced backward. The only way this would happen was if the atom had a small, heavy region of positive charge inside it.