a long-cherished dream of alchemists was to produce gold from cheaper and more abundant elements. this dream was finally realized when 198hg 80 was converted into gold by neutron bombardment. write a balanced equation for this reaction. include both the atomic number and mass number of each species.
Answers
In 1941, gold was created by neutron bombardment from mercury, but the isotopes were all radioactive.
Neutron bombardment: what happens?What is neutron irradiation? When a high-energy neutron bombards a nucleus to start nuclear fission, more neutrons are released during the process. A neutron has no charge, thus when it is pointed at the nucleus, it is not repulsed.
What occurs when uranium is irradiated with neutrons using the mentioned apparatus?When uranium-235 is irradiated with neutrons, the uranium atoms split, releasing heat and atoms with decreasing atomic numbers as well as more neutrons. Similar to typical fossil fuel power plants, this process, known as fission, may harness the heat produced to produce electricity.
To know more about bombardment visit :-
https://brainly.com/question/1103578
#SPJ4
Related Questions
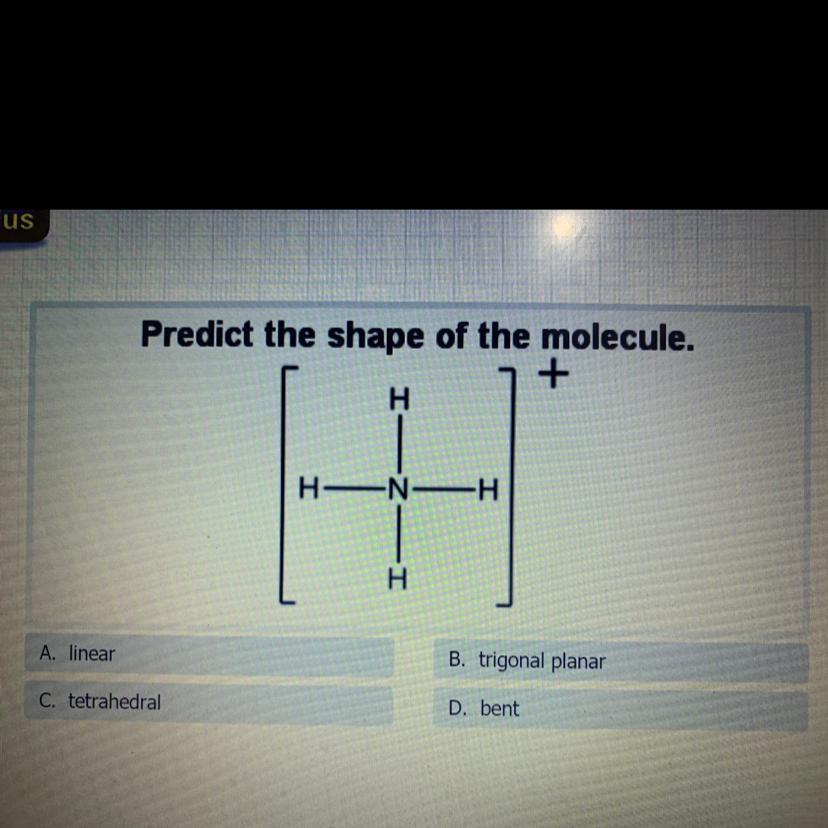
Predict the shape of the molecule.
B. trigonal planar
A. linear
D. bent
C. tetrahedra
Answers
the element hafnium (hf) has five stable isotopes. the correct number of nuclear particles in an atom of hafnium-178 is:
Answers
The correct number of nuclear particles in an atom of hafnium-178 is: 106 protons, 72 neutrons.
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals.
Thus, the correct number of nuclear particles in an atom of hafnium-178 is: 106 protons, 72 neutrons.
To learn more about protons check the link below:
https://brainly.com/question/1805828
#SPJ4
24. what is the most likely method of decay of the radioactive isotope technicium-99 (99tc)? a. alpha decay b. beta decay c. electron capture d. positron emission e. both electron capture and positron emission
Answers
The radioactive isotope Technium-99 decays most likely through alpha decay (99tc). An atomic nucleus emits an alpha particle during the radioactive decay process known as "alpha decay".
and then changes or "decays" into a separate atomic nucleus with a mass number that is decreased by four and an atomic number that is decreased by two. The nucleus of an atom of helium-4 is the same as an alpha particle. Radioisotopes are an element's radioactive isotopes. They are the atoms with unstable neutron-proton combinations or excess energy in their nuclei. During those processes, the radionuclide is said to experience radioactive decay, albeit the surplus energy may be put to use in any number of ways.
Learn more about radioactive isotope here
https://brainly.com/question/1907960
#SPJ4
You work for a custom electrochemical battery company, and they promise customers that they can design a galvanic cell for any target cell voltage. A customer requests the specific voltage of 0.989 V at standard temperature and pressure. How would you create this cell? You can use a maximum electrolte concentration of 2.0 M and any standard half-cell found in the table of standard potentials in your textbook (pg. 875, Petrucci 11th). Explain your design and what you would need to create this cell. Expand on why we might want to design cells with a particular voltage in the real world.
Answers
In order to achieve a target voltage of 0.989 V under standard temperature and pressure conditions, I would carefully choose two half-cells that possess considerably different standard electrode potentials.
How important is this selection?This selection will enable the desired voltage to be reached. Using the standard potentials table from the textbook, I would select an appropriate oxidation half-reaction and a corresponding reduction half-reaction.
One can achieve the desired voltage of a cell by interlinking its half-cells and facilitating the conduction of electrons through an outer circuit.
Crafting cells with precise voltages is crucial in practical usage for numerous reasons.
Certain equipment or mechanisms necessitate a particular voltage for optimal performance. We can make these devices compatible by adjusting the cell voltage according to their requirements.
Additionally, optimized voltage stipulations may be essential to achieve effective energy transformation, particularly in the case of fuel cells or batteries employed in electric cars. By customizing the voltage of the cell, we can enhance the effectiveness of energy storage and usage across different use cases.
Read more about voltage here:
https://brainly.com/question/1176850
#SPJ1
Which of these is a sign of salinization in crop plants?
A. Yellowing of leaves
B. Wilting of seedlings
C. Dead patches on leaves
D. Enlarged seedlings
Answers
Answer:
The correct answer I think is Wilting of Seeds
Explanation:
salinization is when the water evaporates and leaves behind the salt in the water. Which causes the seed to whither because salt sits in its roots, and leaves no way for any more water to get in. So it makes it hard for seedling so get water.
I think that is correct! Hope its right
A sign of salinization in crop plants is Wilting of seedlings. Thus the correct option is B.
What is salinization ?Increased salt percentage in the soil is known as salinization, and it is brought on by soluble substances in the water supply. The land may be flooded by seawater or saline groundwater flowing through the soil downward, depending on the source of the water.
Due to the poor plant growth circumstances caused by salinity, the ground surface is constantly moist and lacks vegetation. Lands become very prone to erosion as a consequence. The amount of salt on the soil's surface rises as a result of excessive irrigation owing to evaporation.
Saline soils cause crops to suffer because of high salinity stress, nutritional problems and toxins, poor soil physical conditions, and decreased agricultural output.
Therefore, option B is appropriate.
Learn more about salinization, here:
https://brainly.com/question/5082030
#SPJ2
what property of dishwashing liquid (detergent) makes it useful to wash grease from pans?
Answers
Dishwashing liquid (detergent) is useful for removing grease from pans due to its amphipathic character.
Grease is a type of liquid lubricant that has thickening agents dispersed throughout it. It can also be solid or semisolid. A soap is typically emulsified with mineral or vegetable oil to form grease.
One typical characteristic of greases is that they have a high initial viscosity, which, when sheared, decreases to create the impression of an oil-lubricated bearing with a viscosity that is roughly equal to the base oil used in the grease. Shear thinning refers to this change in viscosity. Sometimes the term "grease" is used to refer to lubricating substances that are merely soft solids or highly viscous liquids; however, these substances lack the shear-thinning characteristics of traditional grease. For instance, petroleum jellies like Vaseline aren't typically categorized as greases.
Learn more about grease here:
https://brainly.com/question/12045293
#SPJ4
Now, we would like you to share your plant evaluations with Sandy Waters, President of Save the Shoreline. You will do this by writing Mr. Waters a letter. When writing your letter, be sure to use a traditional letter format.
Must include:
an opening that describes the procedure for ranking plants
all plant rankings
evidence from the data to support plant rankings
a closing that explains how plant rankings could protect the dunes
Answers
The given question is incomplete the complete question is
Now, we would like you to share your plant evaluations with Sandy Waters, President of Save the Shoreline. You will do this by writing Mr. Waters a letter. When writing your letter, be sure to use a traditional letter format. This means that your letter should include a greeting (Dear ________, ) a body, and a closing (Sincerely, _________). The body of your letter must include the following: an opening that describes the procedure for ranking plants all plant rankings evidence from the data to support plant rankings a closing that explains how plant rankings could protect the dunes As always, be sure to use complete sentences and proper punctuation, capitalization, and spelling.
Identification of plant includes scientific name and origin of species
Plant is the living thing that grow in the earth and that has a stem leaves and roots
Dear sandy water, the most common process of plant identification is by comparison and in this process the newly collected sample is compared with another collected and identified sample if all characteristics are same then name of sample can be determined
Your sincerely
With this information, we can conclude that identification of a sample includes providing the scientific name and origin of the species
Know more about water
https://brainly.com/question/17898776
#SPJ1
Answer:
Nice Profile Pic
Explanation:
what is geothermal energy ?
And how is it used?
Answers
geothermal energy, form of energy conversion in which heat energy from within Earth is captured and harnessed for cooking, bathing, space heating, electrical power generation, and other uses.
Answer:
it can use for you wanted to do and we can use it for our wants...
Explanation:
⠀⠀◣ ◢
⠀⠀█ █
⠀⠀█ █
⠀⠀◤ ◥
BTS
425,000 mL = __ L in metric conversion
Answers
Answer:
425l
Explanation:
m = 10^-³
425000ml
425000 x 10^-³l
425l
The energy required for ozone destruction reaction O 3
−>O+O 2
is 105 kJ/mol. If photons that have energy greater than 105 kJ/mol, they can induce the reaction. True False Reset Selection
Answers
True. Photons with energy greater than 105 kJ/mol can induce the ozone destruction reaction, causing the O3 molecule to break into O and O2. This is because the energy of the photons is sufficient to overcome the activation energy barrier required for the reaction to occur.
The energy required for the ozone destruction reaction is 105 kJ/mol. If a photon possesses energy greater than this threshold, it can provide the necessary energy for the reaction to take place. When a photon with sufficient energy collides with an ozone molecule (O3), it can transfer its energy to the molecule, breaking it into oxygen atoms (O) and molecular oxygen (O2).
This process is called photodissociation and is responsible for the destruction of ozone in the upper atmosphere. Thus, photons with energy exceeding the activation energy can induce the ozone destruction reaction.
To learn more about Photons click here: brainly.com/question/19385998
#SPJ11
15 ft is the same as how many yards
Answers
8. Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a ___________________ solution.
Answers
Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a hypertonic solution.
What is a hypertonic solution?A hypotonic solution is described as a solution that has lower osmotic pressure than another solution to which it is compared.
The concept of tonicity helps us understand that the saltwater we use to brine the turkey is typically considered hypertonic solution because it has a greater concentration of solutes than the liquid found inside the cells.
Learn more about hypertonic solutions at: https://brainly.com/question/4237735
#SPJ1
Identify the acid associated with each conjugate base. I-SO4 2-Cl-OH -F-
Answers
The acid associated with each conjugate base is I-SO4 2= H2SO4 (sulfuric acid), ClO= HClO(hypochlorous acid), OH= H2O( water) and F- = HF (hydrofluoric acid).
For each conjugate base, the associated acid can be identified by adding a proton (H+) to the anion. The acid associated with each conjugate base is as follows:
I-SO4 2- : The conjugate base is derived from the acid H2SO4, which is sulfuric acid. The acid can donate two protons to form H+ ions and SO4 2- ions in aqueous solution.ClO- : The conjugate base is derived from the acid HClO, which is hypochlorous acid. The acid can donate a proton to form H+ and ClO- ions in aqueous solution.OH- : The conjugate base is derived from the acid H2O, which is water. The acid can donate a proton to form H+ and OH- ions in aqueous solution.F- : The conjugate base is derived from the acid HF, which is hydrofluoric acid. The acid can donate a proton to form H+ and F- ions in aqueous solution.Conjugate acid-base pairs are important in many chemical reactions, including acid-base reactions, redox reactions, and complex formation reactions. In acid-base reactions, for example, the acid donates a proton to the base to form its conjugate base and conjugate acid. The strength of the acid and base determines the position of the equilibrium in the reaction, with stronger acids and bases driving the reaction toward the formation of their weaker conjugate partners.
In summary, the concept of conjugate acids and bases is a fundamental aspect of acid-base chemistry and helps to explain the behavior of acids and bases in many different chemical reactions.
Learn more about conjugate base here:
https://brainly.com/question/30225100
#SPJ4
Gas X has the molecular formula C5Hx. A mass of 1.44g X occupies a volume of 0.32 dm*3 at 2 atm and 400K. Find the value of x. [H=1; C=12; R =0.08 atm.dm3 K*-1.mol*-1]
Answers
The value of x in the gas C₅Hx is 12.
Calculation:-
PV = nRT
n = PV/RT
= 2 × 0.32 / 0.08 × 400
= 0.64 / 32
= 0.02
mole = mass/molar mass
molar mass = mass/ mole
= 1.44/0.02
= 72 grams.
C5Hx = 72
12 × 5 + x = 72
x = 72 - 60
x = 12
An ideal gas is a theoretical gas composed of many randomly transferring factor particles that aren't difficult to interparticle interactions. the best gasoline idea is beneficial because it obeys the precise gas law, a simplified equation of country, and is amenable to evaluation under statistical mechanics.
An ideal gas is described as one for which both the extent of molecules and forces between the molecules are so small that they have got no effect on the behavior of the gas. The real gas that acts almost like a really perfect gasoline is helium. that is due to the fact helium, in contrast to maximum gases, exists as an unmarried atom, which makes the van der Waals dispersion forces as low as viable.
Learn more about ideal gas here:-brainly.com/question/20348074
#SPJ9
what type of chemical bond forms between two atoms bearing opposite charges?
Answers
Ionic bonds are created when two oppositely charged ions are attracted to one another by electrostatic force. A chemical element is uniquely defined by its atoms, which are tiny pieces of substance.
An atom is made up of a core nucleus and one or more negatively charged electrons that orbit it. The positively charged, comparatively hefty protons and neutrons that make up the nucleus may be present. Any elementary particle of matter with at least one proton is referred to as an atom. Examples of atoms are neon (N) and hydrogen (H) (Ne). Chemistry's fundamental building component is an atom. It is the lowest fraction of substance into which electrically charged particles cannot be released. It is also the smallest piece of substance with chemical element-like characteristics.
learn more about atom here:
https://brainly.com/question/13654549
#SPJ4
Use stoichiometry calculations and show all work:
calculate how many grams of PbSO4 would be produced if 5.5 grams of LiNO3 were produced.
Reaction: Li2SO4(aq) + Pb(NO3)(aq) = 2LiNO3(aq) + PbSO4(s)
Answers
Answer:
Explanation:
The reaction you provided is a balanced chemical equation, meaning that for every mole of LiNO3 produced, an equal amount of PbSO4 is produced. However, since you only know the amount of LiNO3 produced (5.5 grams), you can use stoichiometry to find the amount of PbSO4 produced.
First, you need to convert the amount of LiNO3 to moles:
5.5 g LiNO3 / 6.939 g/mol = 0.796 mol LiNO3
Next, using the stoichiometry of the reaction, you can find the number of moles of PbSO4 produced:
0.796 mol LiNO3 * (1 mole PbSO4 / 2 moles LiNO3) = 0.398 moles PbSO4
Finally, you can convert the number of moles of PbSO4 to grams:
0.398 moles PbSO4 * 247.77 g/mol = 98.42 g PbSO4
So, if 5.5 grams of LiNO3 were produced, then 98.42 grams of PbSO4 would be produced.
what happens to the rate of a reaction as the reaction progresses
Answers
Study the reactions for the formation of compounds from their elements. I. C(s) + O2(g) → CO2(g) ΔHf = −394 kJ II. H2(g) + 12O2(g) → H2O(l) ΔHf = −242 kJ III. 2C(s) + 3H2(g) → C2H6(g) ΔH =−84 kJ The combustion of C2H6 is shown by the following equation: C2H6(g) + 72O2(g) → 2CO2(g) + 3H2O(l) Which option correctly gives the enthalpy of combustion of 0.2 moles of C2H6(g)? −1,430 kJ 286 kJ −286 kJ 1,430 kJ Exam 3 Click on the numbers to jump from one question to another. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Answers
Answer:
The correct option is -286 kJ
Explanation:
The given parameters are
C(s) + O₂(g) → CO₂(g) ΔHf = -394 kJ
H₂(g) + 12O₂(g)→H₂O ΔHf = -242 kJ
2C(s) + 3H₂(g)→C₂H₆(g) ΔH = -84 kJ
Te given equation is C₂H₆(g) + 7/2O₂(g) →2CO₂(g) + 3H₂O(l)
The heat of formation or enthalpy of combustion = Heat of formation of the products - Heat of formation of the reactants
The enthalpy of combustion of the reaction = 2*(-394) + 3*(-242)- ((-84)+7/2*0)) = -1,430 kJ
Given that the reaction consists of one mole of C₂H₆(g), we have;
The enthalpy of combustion of one mole of C₂H₆(g) = -1,430 kJ
Therefore, the enthalpy of combustion of 0.2 mole of C₂H₆(g) = -1,430 kJ × 0.2 = -286 kJ
The correct option = -286 kJ.
Answer:
Positive 1,430
Explanation:
under which of the following sets of conditions would a gas sample be least likely to behave ideally?
A. High pressure, low temperature.
B. Low pressure, low temperature.
C. High pressure, high temperature.
D. Low pressure, high temperature.
Answers
High pressure and low temperature conditions are the ones where gas samples are least likely to behave ideally.
Option D. Low pressure, high temperature.
An ideal gas is a theoretical gas composed of a large number of small particles that are in constant random motion. These particles have negligible volume and do not attract or repel each other. Therefore, ideal gases follow the gas laws, which describe the relationship between pressure, volume, temperature, and number of particles.
However, real gases do not always behave ideally. The behavior of real gases is affected by several factors, such as intermolecular forces, particle size, and the volume of the gas particles.
Now coming to your question, under which of the following sets of conditions would a gas sample be least likely to behave ideally? The answer is option A, high pressure and low temperature. This is because at high pressures and low temperatures, the intermolecular forces between the gas particles become more significant, and the volume of the gas particles becomes more significant. These factors cause the gas particles to deviate from ideal gas behavior.
At low pressures and high temperatures (option D), the volume of the gas particles is relatively small compared to the volume of the container, and the intermolecular forces are weak. Therefore, gases are more likely to behave ideally under these conditions.
To know more about ideal gas visit:-
https://brainly.com/question/30236490
#SPJ11
Can we all agree chemistry is hard lol
Answers
Yes, I agree.
Chemistry can be difficult.
Write a Lewis structure for each of the following molecules that are exceptions to the octet rule. Part A BBr3 Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons. To change the symbol of an atom, double-click on the atom and enter the letter of the new atom. Part B NO Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons.
Answers
The total number of valence electrons in BBr3 is 3 + 21 = 24.
The Lewis structure for NO is N = O
How did we arrive at the value?Part A: BBr3
To draw the Lewis structure for BBr3, we first need to determine the total number of valence electrons. Boron (B) is in Group 13, so it has 3 valence electrons, and each bromine (Br) atom has 7 valence electrons. Since there are three bromine atoms, the total number of valence electrons is:
Boron (B) = 3 valence electrons
Bromine (Br) = 7 valence electrons × 3 = 21 valence electrons
Thus, the total number of valence electrons in BBr3 is 3 + 21 = 24.
Now, let's draw the Lewis structure:
Step 1: Place the central atom.
Since boron is less electronegative than bromine, boron should be the central atom. Place the boron (B) atom in the center of the grid.
Br
|
B — Br
|
Br
Step 2: Connect the atoms with bonds.
Each bromine atom needs one bond to satisfy the octet rule. Connect each bromine atom to the central boron atom with a single bond.
Br
|
B — Br
|
Br
Step 3: Distribute the remaining electrons.
We have 24 valence electrons to distribute. Place the remaining electrons as lone pairs around the atoms to complete their octets. Start by placing lone pairs around the terminal atoms (bromine) until they have 8 electrons around them. Then, distribute the remaining electrons as lone pairs around the central atom (boron).
Br
|
B — Br
|
Br
Step 4: Check for octet rule satisfaction.
In the Lewis structure, each bromine atom has a full octet (8 electrons), and the boron atom has only 6 electrons. Boron is an exception to the octet rule and can accommodate only 6 electrons. Therefore, the Lewis structure for BBr3 is:
Br
|
B — Br
|
Br
Part B: NO
To draw the Lewis structure for NO, we follow the same steps as above. The nitrogen (N) atom is less electronegative than oxygen (O), so nitrogen should be the central atom.
Step 1: Place the central atom.
Place the nitrogen (N) atom in the center of the grid.
N = O
Step 2: Connect the atoms with bonds.
Nitrogen needs one bond to satisfy the octet rule. Connect the nitrogen atom to the oxygen atom with a single bond.
N = O
Step 3: Distribute the remaining electrons.
We have 11 valence electrons to distribute. Place the remaining electrons as lone pairs around the atoms to complete their octets. Start by placing lone pairs around the terminal atom (oxygen) until it has 8 electrons around it. Then, distribute the remaining electrons as a lone pair around the central atom (nitrogen).
N = O
Step 4: Check for octet rule satisfaction.
In the Lewis structure, the oxygen atom has a full octet (8 electrons), and the nitrogen atom has only 6 electrons. Nitrogen is an exception to the octet rule and can accommodate only 6 electrons. Therefore, the Lewis structure for NO is:
N = O
learn more about Lewis structure: https://brainly.com/question/20300458
#SPJ4


Density of 1kg of metallic sphere is 2.5 gcm-3 then the volume occupied by sphere is?
Answers
Answer:
400cm^3
Explanation:
d = 2.5gcm^-3
m = 1 kg or 1000g
v = ?
The formula that incorporates all three is:
d = m/v
In order to find out v, rearrange the formula:
v = m/d
Thus,
v = 1000/2.5 = 400cm^3
impact of surface ocean conditions and aerosol provenance on the dissolution of aerosol manganese, cobalt, nickel and lead in seawater
Answers
The dissolution of aerosol manganese, cobalt, nickel, and lead in seawater is influenced by surface ocean conditions and aerosol provenance .
Surface ocean conditions play a significant role in the dissolution of aerosol metals in seawater. Factors such as temperature, pH, salinity, and the presence of other chemical species can affect the solubility and reactivity of metals. For example, higher temperatures and lower pH levels can enhance the dissolution of metals, while increased salinity may decrease their solubility.
Aerosol provenance, which refers to the source and composition of the aerosol particles, also impacts metal dissolution in seawater. Different aerosol sources can have varying mineralogical and chemical compositions, leading to differences in metal solubility and reactivity. Additionally, the size distribution of aerosol particles and their surface properties can influence the rate of metal dissolution.
Understanding the impact of surface ocean conditions and aerosol provenance on metal dissolution is crucial for assessing the fate and transport of metals in marine environments. It helps in studying their bioavailability, potential toxicity, and ecological implications.
Learn more about dissolution from the given link https://brainly.com/question/31981760
#SPJ11
the arrangement of electrons in an atom of an element determines the chemical properties of that element. Our present day understanding of how electrons are arranged in an atom is the result of all of these scientific contributions except
Answers
The result of all of these scientific contributions except Rutherford's gold foil experiment proved the existence of the nucleus.
The valence electrons mainly determine the chemical houses of the elements. The elements inside the equal group have comparable chemical homes because they have identical valence shell electron configurations. The electrons in the power degrees with the highest range are in common the farthest from the nucleus.
Because variations in electrons placed inside the outermost stage distinguish an atom from its nearest noble fuel these are the electrons accountable for the atom's chemical conduct. Electrons located inside the outermost shell of the electron cloud are called valence electrons and feature the best power. Valence electrons determine the chemical homes of an element or how the valence electrons of 1 element are shared or traded with valence electrons of other elements to create new molecules.
Learn more about Electrons here:-https://brainly.com/question/860094
#SPJ4
why does ionization energy increase across a period?
Answers
Answer: I
I
\/
Explanation: In general, ionization energy increases across a period and decreases down a group. Across a period, effective nuclear charge increases as electron shielding remains constant. ... The increased distance weakens the nuclear attraction to the outer-most electron, and is easier to remove (requires less energy).
The maximum number of electrons that can be present in an SHELL is
a) \(2 {n}^{2} \)
b) \(2 {n}^{2} + 1\)
c) 2n
d) none of these.
Answers
\(\huge\boxed{\fcolorbox{red}{blue}{ QUESTION }}\)
The maximum number of electrons that can be present in an shell is
\( \orange{\underline{\huge{\bold{\textit{\green{\bf{OPTIONS}}}}}}} \)
a) \(2 {n}^{2} \)
b) \(2 {n}^{2} + 1\)
c) 2n
d) none of these.
\( \huge\mathbb{\red A \pink{N}\purple{S} \blue{W} \orange{ER}}\)
OPTION (a)
\(2 {n}^{2} \)
\( \bold { \red{ \star{ \blue{EXAMPLES}}}}\)
In First shell only 2 electron can be placed .
\(2 {n}^{2} \\ n = 1 \: (bez \: \: no \: \: shell \: = 1) \\ so \\ 2 { n}^{2} = 2 \times {1}^{2} = 2 \: electrons\)
In second shell 8 electrons can be placed
\(2 {n}^{2} \\ n = 1(bez \: \: no\: \: shell \: = 2) \\ 2 {n}^{2} = 2 \times {2}^{2} = 2 \times 4 = 8 \: electrons\)
This column of the periodic table represents the halogen family. This is a family of reactive elements. Compare
and contrast how the elements are similar and different.
A)
The mass numbers differ; they have the same number of protons
and valence electrons.
B)
They have different atomic numbers and mass numbers; they
have the same number of electrons.
C)
They have different atomic numbers and mass numbers, they
have the same number of valence electrons.
D)
They have different numbers of protons, neutrons, and valence
electrons: they are all gases at room temperature.
Answers
Answer:
C
Explanation:
I took it on usatestprep
Write the ionization reaction of hydrochloric acid. note: mention the oxidation states along with each element.
Answers
The ionization reaction of hydrochloric acid (HCl) can be represented as follows:HCl → H+ + Cl-The oxidation state of hydrogen in hydrochloric acid is +1, while that of chlorine is -1. When hydrochloric acid dissolves in water, it forms hydronium ions (H3O+).
The ionization reaction of hydrochloric acid is: HCl → H+ + Cl-. The oxidation state of hydrogen in HCl is +1, and that of chlorine is -1. Hydrochloric acid (HCl) is an aqueous solution of hydrogen chloride gas. It is a strong acid that is completely dissociated in water to form hydronium (H3O+) and chloride (Cl-) ions.
The reaction is an example of a simple acid-base reaction. The oxidation state of hydrogen in hydrochloric acid is +1, while that of chlorine is -1.The ionization reaction of hydrochloric acid is:HCl → H+ + Cl-The oxidation state of hydrogen in HCl is +1, and that of chlorine is -1.
To know more about acid visit.
https://brainly.com/question/29796621
#SPJ11
Calculate the mass of water that contains same number of hydrogen atoms as 34 g of ammonia.
Answers
The mass of water that contains same number of hydrogen atoms as 34 g of ammonia is 54 g.
First calculate the number of moles in a hydrogen gas. The molar mass of ammonia(NH₃) is 17 g/mol. Hence, the number of moles for ammonia(NH₃) is
moles=34 g×(1 mol/17 g)
=2 mol
The relation between moles and number of atoms is given as
1 mol=6.022×10²³ atoms
Since 1 mol NH₃ contains 3 mol of hydrogen. Hence, the number of hydrogen atoms in 2 mol NH₃ is
2 mol NH₃×(3 mol H/1 mol N)×(6.022×10²³ atoms/1 mol H)
=(2×3×6.022×10²³ atoms)
=3.6132×10²⁴ atoms
The molecular formula of water is H₂O. First, we need to find out the number of moles in the calculated atoms of hydrogen. One mol of water contains 2 moles of H. Hence, the number of moles of water is
3.6132×10²⁴ atoms×(1 mol H/6.022×10²³ atoms)×(1 mol H₂O/2 mol H)
=(3.6132×10²⁴ mol of H₂O/6.022×10²³×2)
=(3.6132×10²⁴ mol of H₂O/1.2044×10²⁴)
=3 mol of H₂O
The molar mass of of H₂O(water) is 18 g/mol. Hence, the mass of water is
3 mol H₂O×(18 g H₂O/1 mol H₂O)
=54 g H₂O.
Therefore, the mass of water which contains the same number of hydrogen atoms as 34 g of ammonia is 54 g of H₂O.
To know more about the number of atoms
https://brainly.com/question/20038872
#SPJ4
a chain-like molecule composed of repeating sub-units is known as a
Answers
A chain-like molecule composed of repeating sub-units is known as a polymer. Polymers are formed by linking smaller units called monomers together through covalent bonds.
The repeating structure of the monomers in a polymer chain gives it its characteristic properties. Polymers can have a wide range of properties depending on the composition and arrangement of the monomers. They can be flexible or rigid, transparent or opaque, and can have varying mechanical, thermal, and electrical properties.
Polymers find applications in numerous industries, including packaging, textiles, construction, automotive, and healthcare. Their versatility and customizable properties make them invaluable materials in modern society.
Overall, the chain-like structure of polymers allows for a remarkable range of properties and applications, making them an essential class of materials in various industries.
Learn more about chain-like molecule here : brainly.com/question/15549326
#SPJ11