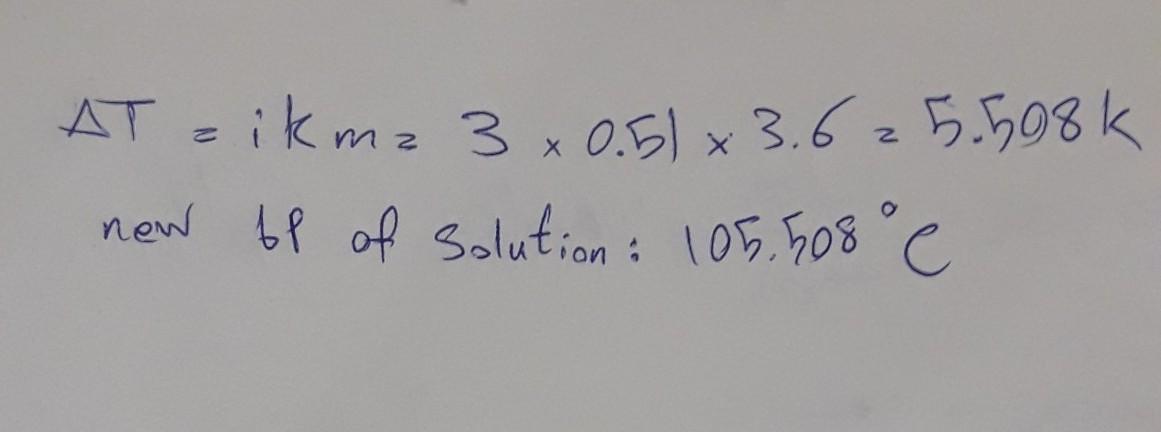Answers
A liquid has a volume of 4 mL and a mass of 24 g, 6gm/mol is the density of the liquid.
What is density?Density is the mass of a material substance per unit volume. d = M/V, where d is density, M is mass, and V is volume, is the formula for density. The unit of density that is most frequently used is grams per cubic centimeter, or gm/mol.
One of an object's most significant and practicable physical characteristics is density. Densities are often used to identify pure substances, classify mixtures, and estimate their composition.
To calculate volume of a substance, we use the equation:
Density = Mass/volume
We are given:
Mass of liquid = 24 g
Volume of liquid = 4 ml
Putting values in above equation, we get:
density = 24 gm /4 ml
or, density = 6 gm/mol.
Hence, the density of liquid is 6 g/ml
To know more about density refer to:
https://brainly.com/question/1354972
#SPJ1
Related Questions
President Bush signed the Energy Policy Act in 2005. How did that affect hydropower?
Answers
President Bush signed the Energy Policy Act in 2005 affect hydropower because Bush enacts the Energy Policy Act as law in an effort to address the rising energy issues. By offering tax breaks and credit guarantees for energy production of all kinds, including hydropower, the act altered American energy policy.
What is hydropower about?The revolutionary Architecture Hydraulique, written by French hydraulic and military engineer Bernard Forest de Bélidor, marked the beginning of the development of the modern hydropower turbine.
Note that the Energy Policy Act of 2005 was signed by President Bush on August 8, 2005. The Underground Storage Tank Compliance Act of 2005, Title XV, Subtitle B, amends Subtitle I of the Solid Waste Disposal Act, the primary law that established the underground storage tank (UST) program.
Learn more about hydropower from
https://brainly.com/question/8934285
#SPJ1
Calculate the activation energy in kJ/mol using data you calculated for rate constant at room temperature and cold temperature. ( R=8.314 J mol-1 K-1) Use the rearranged Arrhenius equation provided in the introduction for this calculation. Use T1 as your cold temperature and T2 as your room temperature, and k1 and k2 respectively. NOTE: Refer equation (4)
1. Rate Constant at room temperature:
2. Rate Constant at cold temperature
3. Temperature of the reaction mixture at room temperature
4. Temperature of the reaction mixture at cold temperature
5. Activation energy
Answers
The activation energy (Ea) in kJ/mol is equal to the product of the rearranged Arrhenius equation multiplied by 1000 kJ/mol.
To calculate the activation energy (Ea) in kJ/mol, you can use the rearranged Arrhenius equation (Equation 4):
Ea = R * (1/T2 - 1/T1) * ln(k2/k1)
Given:
k1 = Rate Constant at cold temperature
k2 = Rate Constant at room temperature
T1 = Temperature of the reaction mixture at cold temperature
T2 = Temperature of the reaction mixture at room temperature
R = 8.314 J mol-1 K-1
Calculation:
Ea = 8.314 J mol-1 K-1 * (1/T2 - 1/T1) * ln(k2/k1)
Ea = (8.314 J mol-1 K-1 * (1/T2 - 1/T1)) * ln(k2/k1)
Ea = (8.314 J mol-1 K-1 / T2 - 8.314 J mol-1 K-1 / T1) * ln(k2/k1)
Ea = (8.314 J mol-1 K-1 / T2 - 8.314 J mol-1 K-1 / T1) * ln(k2/k1)
Ea = (8.314 J mol-1 K-1 / T2 - 8.314 J mol-1 K-1 / T1) * ln(k2/k1) * 1000 kJ/mol
Ea = (8.314 J mol-1 K-1 / T2 - 8.314 J mol-1 K-1 / T1) * ln(k2/k1) * 1000 kJ/mol
Here you can learn more about Arrhenius equation
https://brainly.com/question/12907018#
#SPJ11
Most cooking utensils are made up of aluminum because aluminum is _____
Answers
Explanation:
Cooking utensils, such as pots, pans and menu trays, are often made from aluminium because it is lightweight and conducts heat well, making it energy-efficient for heating and cooling. These properties also make it a preferred material for packaging.
Calculate the volume in milliliters of a 0.595M iron(II) bromide solution that contains 100. g of iron(II) bromide . Round your answer to significant digits.
Answers
The volume, in milliliters of 0.595 M iron (II) bromide that will contain 100 g of the substance will be 779.3 mL.
Molarity problemThe molarity of a solution is the ratio of the mole of solutes to the volume of the solution. Mathematically:
Molarity = mole/volume
Also, mole = mass/molar mass
Thus, 100 g of iron (II) bromide (molar mass = 215.65 g/mol) would be:
100/215.65 = 0.4637 mol
Making the volume of the subject from the equation above:
Volume = mole/molarity
= 0.4637/0.595
= 0.7793 liters
In milliliters, 0.7793 liters = 0.7793 x 1000 = 779.3 mL
Thus, the volume of the solution would be 779.3 mL.
More on molarity-related problems can be found here: https://brainly.com/question/15421287
#SPJ1
Consider the two electron arrangements for neutral atoms A and B. What is the atomic number of A?
A - 1s?, 2s 2p6,35
B - 1s2, 2s 2p, 5s
Answers
Which of the following has a fixed volume and shape?
milk feather carbon dioxide oxygen
Answers
Answer:
the feather
Explanation:
solids do not change shape
safety rules for using primus stove
Answers
Answer:
1. Never leave the gas cartridge in the stove when you’re not using it.
2. Always wait until the stove cools down before you remove the gas cartridge.
3. Always try to store gas cartridges and camping stoves in a dry place
4. Clean your stove each time you have used it because it needs to be clean in order to perform at its best.
Atomic number of a member of halogens
Answers
Chlorine 17
Flourine 9
Iodine 53
Astatine 85
22.55 mL of an H2SO4 solution
were titrated with 14.85 mL of a
0.146 M NaOH solution to reach the
equivalence point. What is the
molarity of the H2SO4 solution?

Answers
The concentration of H₂SO₄ solution is equal to 0.0480 M.
What is a neutralization reaction?A neutralization reaction is described as a chemical reaction where acid and base react to produce respective salt and water. When a strong acid reacts with a strong base then the salt can be neutral.
When H₂SO₄ (a strong acid) reacts with NaOH, the resulting salt is Na₂SO₃ and water.
H₂SO₄ + 2 NaOH → Na₂SO₄ + 2H₂O
Given, the concentration of NaOH = 0.146 M
The volume of the NaOH = 14.85 ml = 0.01485 L
The number of moles of NaOH, n = M × V = 0.146 × 0.01485 = 0.00216 M
The volume of the H₂SO₄ = 22.55 ml = 0.02255 L
The number of moles of H₂SO₄, n = 0.00216/2 = 0.00108 mol
The concentration of H₂SO₄ =0.00108/0.02255 = 0.0480 M
Therefore, the molarity of H₂SO₄ is 0.0480 M.
Learn more about neutralization reaction, here:
brainly.com/question/20038776
#SPJ1
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
A population’s carrying capacity is the number of individuals that die over a given time period. True or false
Answers
Answer:
I believe its false
Explanation:
a carrying capacity is the maximum amount of living things that can be held in a single container
Answer:
Populations vary in their capacity to grow. The maximum rate at which a population can increase when resources are unlimited and environmental conditions are ideal is termed the population's biotic potential. Each species will have a different biotic potential due to variations in
the species' reproductive span (how long an individual is capable of reproducing)
the frequency of reproduction (how often an individual can reproduce)
"litter size" (how many offspring are born each time)
survival rate (how many offspring survive to reproductive age)
There are always limits to population growth in nature. Populations cannot grow exponentially indefinitely. Exploding populations always reach a size limit imposed by the shortage of one or more factors such as water, space, and nutrients or by adverse conditions such as disease, drought and temperature extremes. The factors which act jointly to limit a population's growth are termed the environmental resistance. The interplay of biotic potential and density-dependent environmental resistance keeps a population in balance.
The density of a sample of NH3(g) at a pressure of 1.00 atm is 0.869 g/L. What is the square velocity (in m/s) of the molecules in this sample?
Answers
The density of a sample of NH\(_3\)(g) at a pressure of 1.00 atm is 0.869 g/L. 592m/s is the square velocity (in m/s) of the molecules in this sample.
The velocity of a gas molecule at a certain temperature, which corresponds to point P in the graph, is 200 ms. At the given temperature, the rms velocity of a gas molecule is about. Molecular count (A) 163ms (C) 245ms (B) 217 milliseconds; (D) 226 milliseconds. The rms velocity of a particular amount of gas molecules at 27oC and 1.0 105 Nm⁻² pressure is 200 msec-1.
velocity = √3p/density
=√3/0.869
=592m/s
To know more about velocity, here:
https://brainly.com/question/24259848
#SPJ1
A student performs an experiment to determine the molar mass of magnesium oxide. The student repeats the experiment five times and collects the following data: 40.220gmol, 40.654gmol, 40.314gmol, 40.165gmol, and 40.554gmol. What is the relative standard deviation (RSD) for this set of data?
Answers
The relative standard deviation for the set of data obtained by the student is 0.470%
The formula for calculating the relative standard deviation is given below as:
Relative standard deviation (RSD) = (S * 100)/x
where S stands for the standard deviation and x is the mean of the data set.
The following are the steps to calculate to determine the relative standard deviation using the given formula:
Step 1: Calculate the mean of the numbers in the data set.
mean, x = sum of numbers / number of numbers
(40.220 + 40.654 + 40.314 + 40.165 + 40.554)/5
mean = 40.381 g/mol
Step 2: Subtract the mean from each number in the data to determine the deviation for each number and then square the deviations.
(40.220 - 40.381)² = 0.0259
(40.654 - 40.381)² = 0.0745
(40.314 - 40.381)² = 0.00449
(40.165 - 40.381)² = 0.0466
(40.554 -40.381)² = 0.0299
Step 3: Sum the squared deviations.
0.0259 + 0.0745 + 0.00449 + 0.0466 + 0.0299 = 0.181
Step 4: Determine the variance by dividing the sum of the squared deviations by the total number of values.
Variance = 0.181/5 = 0.0362
Step 6: Determine the standard deviation of the data by taking square root of the variance.
Standard deviation, S = √0.0362 = 0.190
Step 7: Determine the relative standard deviation by inserting the values in the formula, (RSD) = (S * 100)/x
RSD = (0.190 * 100)/40.381
RSD = 0.470%
Learn more at: https://brainly.com/question/475676
6. In the equation for an enzymatic reaction, ES represents the complex formed between the substrate S and the enzyme protein E. In the final step of the following oxidation reaction, the product P dissociates from the ESO₂ complex, which regenerates the active enzyme: E+S ES K₁ ES+O₂ ESO₂ K₂ ESO₂=E+P K3 Give the overall reaction equation and show that K=K₁XK₂xK₂ (E.1) (E.2) (E.3)
Answers
It is shown that rate equation for enzymatic reaction is proportional to the product of concentrations of enzyme E and enzyme-substrate-oxygen complex ESO₂ and constant of proportionality k' is equal to k₃ multiplied by the product of equilibrium constants K₁ and K₂. This is expressed mathematically as K = K₁ x K₂ x K₃.
What is enzymatic reactions?A physiologically relevant biochemical stimulus for the temporal and spatial control of drug release is called enzymatic reaction .
The overall reaction equation for the enzymatic oxidation reaction is:
S + O₂ → P
S is substrate, O₂ is oxidizing agent, and P is product of the reaction.
The reaction proceeds through the formation of the enzyme-substrate complex (ES), followed by the formation of the enzyme-substrate-oxygen complex (ESO₂), and finally, the dissociation of the product P from the ESO₂ complex, which regenerates the active enzyme E.
Rate equation for this reaction is: v = k₃[ESO₂]
v is rate of the reaction, k₃ is rate constant for the final step, and [ESO₂] is concentration of the enzyme-substrate-oxygen complex.
K₁ = [ES][S] / [E][S] (E.1)
K₂ = [ESO₂][E][O₂] / [ES][E][O₂] (E.2)
K₂ = [ESO₂] / [ES]
K₁ x K₂ = ([ES][S] / [E][S]) x ([ESO₂] / [ES])
K₁ x K₂ = [ESO₂] / [E]
[E][ESO₂] = K₁ x K₂ x [ES]
[ES] = ([E][ESO₂]) / (K₁ x K₂)
v = k₃([E][ESO₂]) / (K₁ x K₂)
v = k'([E][ESO₂])
where k' = k₃ / (K₁ x K₂)
Therefore, we have shown that the rate equation for the enzymatic reaction is proportional to the product of the concentrations of the enzyme E and the enzyme-substrate-oxygen complex ESO₂, and the constant of proportionality k' is equal to k₃ multiplied by the product of the equilibrium constants K₁ and K₂. This is expressed mathematically as K = K₁ x K₂ x K₃.
To know more about enzymatic reactions, refer
https://brainly.com/question/11276447
#SPJ1
46 g of glycerin were dissolved in 100 g of water. What is the freezing point of this solution?
Additional information:
М(С3Н5(ОН)3) = 92 g/mol;
Тf(Н2О) = 273.15 К;
Кf = 1.86 kg⋅К/mol.
Answers
Based on the formula to determine the freezing point depression of the solvent, the freezing point of the solution is 263.85 K.
What is the freezing point of a substance?
The freezing point of a substance is the temperature at which the liquid changes to solid without any further decrease in temperature occurring during the process.
The addition of solute substances in liquids usually lowers the freezing point of the liquid solvent.
The formula to determine the freezing point depression of solvent is given below:
ΔT = i * Kf * mwhere'
ΔT is the change in freezing point,i is the van't Hoff factor,Kf is the freezing point depression constant, andm is the molality of the solution.The molality of the given solution = moles of solute/kg of solvent
moles of solute = 46/92
mass of solvent = 100 g or 0.1 kg
Molality of solution = (46/92) / 0.1
Molality of solution = 5
for glycerine, i = 1
ΔT = ΔT = 1 * 1.86 * 5
ΔT = 9.3
The freezing point of the solution = 273.15 - 9.3
The freezing point of the solution = 263.85 K
Learn more about freezing point depression at: https://brainly.com/question/30093044
#SPJ1
HELPP!!!
Convert 2.75 x 10^4 kJ to calories
Answers
Answer:
6.573
Explanation:
2.7500 x 1cal/4.184kj x 1Cal/1000cal = 6.573
Which part of a seed plant develops into sperm cells? o embryo pollen 0 seed O xylem
Answers
Answer:
Pollen
Explanation:
bc i take notes and also my sister studies bio
PLZ GIMME BRAINLIEST
Answer:
correct
Explanation:
Use the band of stability to determine if europium-154 is a stable or unstable nucleus. Hint: You
must first find the atomic number to determine the number of protons and then use the equation,
neutrons = mass number - protons, to find the neutrons.
On/Z=0.69, unstable
On/Z=0.69, stable
On/Z=1.44, unstable
On/Z=1.44, stable
Answers
Answer:
1.44 STABLE
Explanation:
Why do elements not have a numerical value for standard heats of formation and Free energies of formation but do have a numerical value for standard molar entropies?
Answers
Because it takes no energy to generate a naturally occurring compound, the enthalpy of formation for an element in its elemental state will always be 0.
What do you mean by formation standard free energies?The free energy shift that happens when 1 mole of a material is created from its component elements in their standard states is referred to as the standard free energy of formation. The standard free energy of production of a pure element in its standard state is zero.
The distinction between Gibbs free energy and standard free energy is that the former is dependent on the experimental circumstances, whilst the latter describes the Gibbs free energy for reactants and products in their standard state.
learn more about standard free energy
https://brainly.com/question/14415025
#SPJ1
1. Cells must let what into the cell
Answers
Where is ocean water the densest?
1.The surface
2.The bottom
3.The middle
4.Lake Michigan
Answers
Answer:
your answer should be the bottom
sorry if im wrong
Explanation:
30 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
how many grams of oxygen can be prepared by the decomposition of 12 grams of mercury oxide
Answers
Taking into account the reaction stoichiometry, 0.886 grams of O₂ can be prepared by the decomposition of 12 grams of mercury oxide.
Reaction stoichiometryIn first place, the balanced reaction of the decomposition of mercury oxide is:
2 HgO → 2 Hg + O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
HgO: 2 moles Hg: 2 moles O₂: 1 moleThe molar mass of the compounds is:
HgO: 216.59 g/moleHg: 200.59 g/moleO₂: 32 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
HgO: 2 moles ×216.59 g/mole= 433.18 grams
Hg: 2 moles ×200.59 g/mole= 401.18 grams
O₂: 1 mole ×32 g/mole= 32 grams
Mass of oxygen formedThe following rule of three can be applied: if by reaction stoichiometry 433.18 grams of HgO form 32 grams of O₂, 12 grams of HgO form how much mass of O₂?
\(mass of O_{2} =\frac{12 grams of HgOx 32 grams of O_{2}}{433.18 grams of HgO}\)
mass of O₂= 0.886 grams
Then, 0.886 grams of O₂ can be prepared by the decomposition of 12 grams of mercury oxide.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
Wine goes bad soon after opening because the ethanol dissolved in it reacts with oxygen gas to form water and aqueous acetic acid , the main ingredient in vinegar. Calculate the moles of water produced by the reaction of of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to significant digits.
Answers
Answer:
1.7 moles of ethanol would be needed.
Explanation:
* Calculate the moles of ethanol needed to produce 1.70mol of water. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
First off, we have to state the equation for the reaction.
So we know that;
ethanol + oxygen → acetic acid + water
This leads us to;
C2H5OH + O2 → CH3COOH + H2O
1 1 1 1
To obtain the moles of ethanol needed to produce 1.70mol of water, we look at the stoichiometry of the reaction above.
1 mol of ethanol produces 1 mole of water
x mol of thanol would produce 1.7 mol of water
Thus we have;
1 = 1
x = 1.7
x = 1.7 moles of ethanol would be needed.
Describe four real life examples of the results of scientific investigation
Answers
The findings of scientific investigation can be seen in four real-world situations:
Pluto to not be a planet,compost,surgery,illness remediesExplain about the scientific investigation?Finding the answer to a topic through a variety of research techniques is the process of conducting a scientific investigation.
An investigation typically starts when a person analyzes their surroundings and poses questions they are unsure of the answers to. After that, they conduct additional observations or design an experiment to verify a theory. Observational research in science can involve, for instance, describing in-depth observations of a cell under a microscope. Some scientific studies are experimental; an illustration would be administering a chemical to a cell while observing changes in the conduct of the cell.Thus, the findings of scientific investigation can be seen in four real-world situations:
Pluto to not be a planet,compost,surgery,illness remediesTo know more about the scientific investigation, here
https://brainly.com/question/17571192
#SPJ1
0.4g divalent metal (eq.wt=20) is dissolved in 50cc of 0.64N HCL solution.If further 109.2 cc of NaOH is required to neutralize the resultant solution determine the strength of solution NaOH in g/l ?
Answers
An atomic mass is the mass of a chemical element's single atom. It involves the masses of the three atomic subatomic particles: protons, neutrons and electrons.
Volume of Naoh(V1)= 30cc
Normality of Naoh(N1)= 1N
Volume of excess Hcl(V2) = ??
Normality of Hcl(N2) = molarity × basicity
= 2×2 = 4 × 1.01 = 4.04N
Using,
V1N1 = V2N2
30×1 = V2 × 4.04
I.e. V2 = 7.42cc
Volume of excess acid = 7.42 cc
Volume of Hcl neutralized by metal= 100 - 7.42
= 92.575 cc
Therefore, gm.eqv of Hcl = gm.eqv of metal
(92.575/1000) × 4.04 = 4/E
I.e E = 10.09
Atomic mass= E × basicity
= 10.09 × 2
= 20.18amu ans
The atomic mass is 20.18 amu
Learn more about atomic mass here
https://brainly.com/question/3187640
#SPJ9
Express your answer as a chemical equation. Enter and no reaction if no participate is formed

Answers
From the balanced chemical equation above, we can see that:
• A precipitate may form wh ( BaSO4(s) ) forms.
,• Therefore, ,there is a reaction, that took place here.
Read the given equation:
NH + HCI - NH4ACI
Which of the following is true about the equation?
NH3 is the acid and NH4Cl is the salt.
NH3 is the base and NH4Cl is the salt.
HCI is the acid and NH3 is the salt.
HCl is the base and NH3 is the salt.
Answers
Answer:
NH3 is the base and NH4Cl is the salt.
Explanation:
Hello there!
In this case, according to the given information, it is possible for us to rewrite the chemical equation and thus obtain:
\(NH_3+HCl\rightarrow NH_4Cl\)
Whereas it is possible to notice that ammonia, NH3, received the hydrogen ions from HCl to form NH4 ions and Cl ions; in such a way, we infer that NH3 is the base and NH4Cl is the salt.
Regards!
Answer:
NH3 is the base and NH4Cl is the salt
Explanation:
A or B or C or D, plz answer fassttt

Answers
Answer:
A.
Explanation:
They'd probably want to know the conditions that usually produce fog
What is the boiling point in °C of a 3.6 molal solution of calcium chloride in water?

Answers
Answer:
CaCl2--->Ca2+ + 2Cl_ so i=3