A lead ball is added to a graduated cylinder containing 31.8 mL of water, causing the level of the water to increase to 93.7 mL. What is the volume in milliliters of the lead ball?
a) 31.8 mL
b) 61.9 mL
c) 93.7 mL
d) 125.5 mL
Answers
Given that a lead ball is added to a graduated cylinder containing 31.8 mL of water, causing the level of the water to increase to 93.7 mL. We need to find the volume in milliliters of the lead ball
. We know that the volume of water displaced by the ball is the same as the volume of the ball. So, to find the volume of the ball, we need to subtract the initial volume of water from the final volume of water
. Hence, the main answer is option b) 61.9 : The volume of the lead ball = Final volume of water - Initial volume of waterVolume of the lead ball = 93.7 mL - 31.8 mL= 61.9 mLTherefore, the volume of the lead ball is 61.9 mL
To know more about water visit:
https://brainly.com/question/28465561
#SPJ11
Related Questions
Define: Conversion factors
Answers
an arithmetical multiplier for converting a set of units to another set of units. For example, a foot is 12 inches, so if you were to convert three feet to inches the equation would be 3ft/Xin and you would multiply three by twelve to get 36 inches.
A conversion factor is a number used to change one set of units to another, by multiplying or dividing. When a conversion is necessary, the appropriate conversion factor to an equal value must be used. For example, to convert inches to feet, the appropriate conversion value is 12 inches equal 1 foot.
the overall take away of an experiment’s results is the______. A. hypothesis B. introduction C. conclusion
Answers
Answer:
conclusion
Explanation:
it can't be a hypothesis since tests are carried out to verify so it is not a theory
an introduction to an experiment only gives the basis of what we are investigating therefore nothing has been proven and the question is still unanswered
The overall takeaway of an experiment’s results is the conclusion. Hence, option C is correct.
What is a hypothesis?A hypothesis is a testable statement about the relationship between two or more variables or a proposed explanation for some observed experiment.
The overall takeaway of an experiment’s results can't be a hypothesis since tests are carried out to verify so it is not a theory
An experiment only gives an idea about the investigation therefore nothing has been proven and the question is still unanswered.
Hence, the overall takeaway of an experiment’s results is the conclusion.
Learn more about the hypothesis here:
brainly.com/question/5177511
#SPJ5
All samples of a specific substanice have the same chemical
composition
TRUE
FALSE

Answers
Answer:
true
Explanation:
Write a proposed answer to the question below. In your answer, include what is happening with the molecules.
Question: What happens to the molecules of two liquids when you mix them together?
Answers
The molecules of two liquids mix together and form a new liquid. However, the liquid formed might be miscible or immiscible.
When two liquids are mixed together it forms a new solution. The new liquid has the properties of both of the original liquids.
The formed liquid may either be miscible or immiscible depending upon the properties of the two liquids.
Miscible liquids become homogeneous solutions so that their composition would be uniform. This is done by the complete diffusion of both liquids.
But in the case of immiscible liquids, the solution forms two separate layers giving out heterogeneous solutions.
Therefore, the combination of 2 liquids gives a new solution that could either be homogeneous or heterogeneous.
To know more about liquids, click below:
https://brainly.com/question/752663
#SPJ1
what is the smallest particle which prossesses the properties of a compound called?
Answers
Answer:
Molecule
Explanation:
Compounds are chemical substances comprising of two or more elements. The smallest particle contained in a compound is the MOLECULE. Molecules are combinations of atoms (same or different) of an element held together by a chemical bond.
Since a compound is a molecule with two or more elements combined, the chemistry of the molecules contained in a compound determines its chemical properties. This means that the molecules of a compound are responsible for the interactions of a compound in a chemical reaction. Examples of molecules are H2O, N2, O3 etc.
12. What are two different ways to turn a turbine to generate electricity without using fossil
fuels?
HELPP
Answers
Answer:
two different ways are 1st by using water and 2nd by using wind these also not harm our environment
I'm confused what valence electrons are ? idk if they're the same as just regular electrons
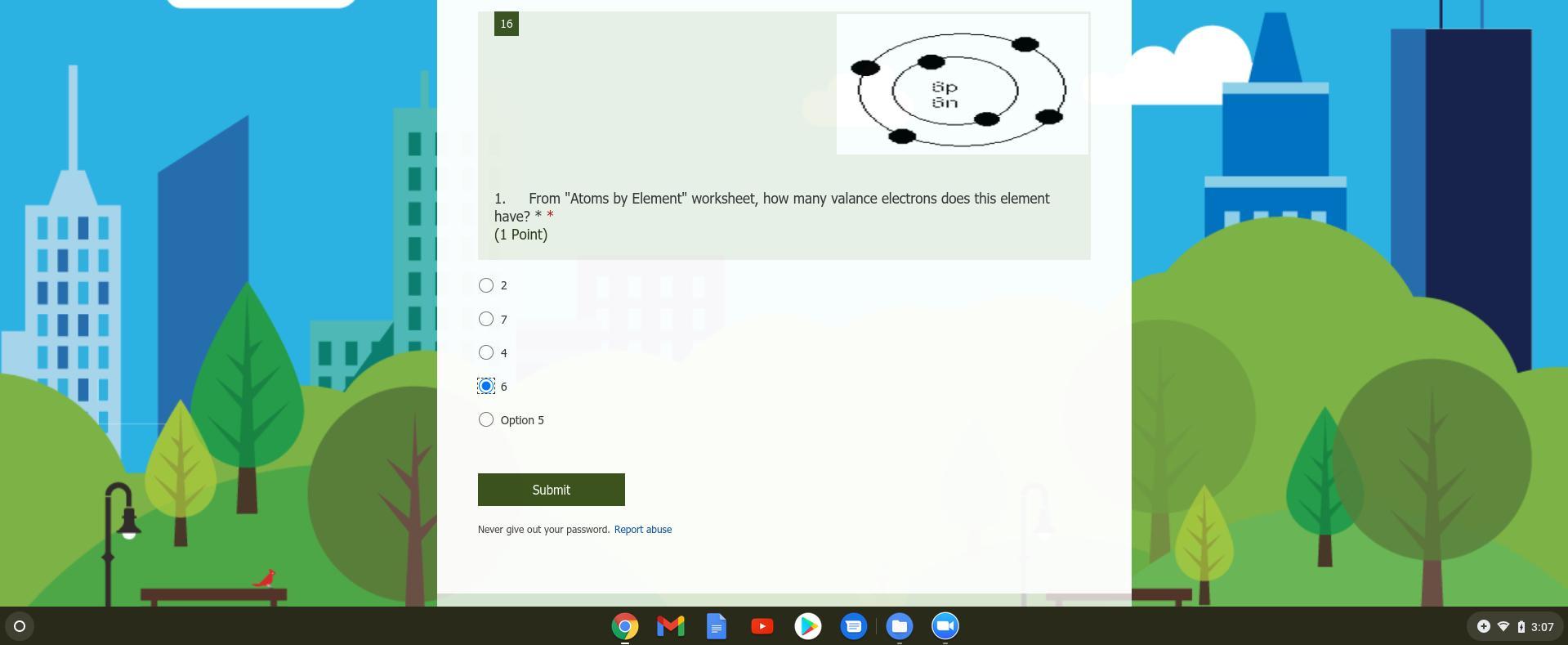
Answers
Answer: valence electrons are simply the electrons in the outer most ring of the chart. in your example, this atom has four valence electrons.
Use the References to access important values if needed for this question. Enter electrons as e-.
A voltaic cell is constructed from a standard Pb2+|Pb Half cell (E° red = -0.126V) and a standard F2|F- half cell (E° red = 2.870V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.)
The anode reaction is:___________
The cathode reaction is:__________
The spontaneous cell reaction is:__________
The cell voltage is ___________V
Answers
We know the standard reduction potentials of the half-cells involved, so we can find the cell voltage and the spontaneous reaction. Thus;
The anode reaction is:
Pb(s) → Pb2+(aq) + 2e-
This is the oxidation half-reaction that occurs in the Pb half-cell.
The cathode reaction is:F2(g) + 2e- → 2F-(aq).
This is the reduction half-reaction that occurs in the F2 half-cell.
The spontaneous cell reaction is
:Pb(s) + F2(g) → Pb2+(aq) + 2F-(aq).
This is the combination of the oxidation and reduction half-reactions, with the electrons canceled out from both sides.
The cell voltage is 2.996 V The standard cell potential is calculated as follows:
standard cell potential = E°(reduction) - E°(oxidation)standard cell potential = 2.870 V - (-0.126 V)standard cell potential = 2.996 V, The cell voltage is positive, indicating that the reaction is spontaneous.
To know more about oxidation half-reaction visit;
https://brainly.com/question/12686471
#SPJ11
Calculate the pH of the following solutions in which the [H+] is a) 0.01, b) 1.0 x 10 –8 , and c) 4.67 x 10 –4
Answers
Therefore, the pH value of the solutions with [H+] values of 0.01, 1.0 x 10 –8, and 4.67 x 10 –4 are 2.0, 8.0, and 3.33 respectively.
pH is a measure of acidity or alkalinity of a solution and ranges from 0 to 14.
The value of pH is defined as the negative logarithm of hydrogen ion concentration. The hydrogen ion concentration in a solution is a measure of its acidity or basicity. The higher the hydrogen ion concentration, the lower the pH value, and the solution is more acidic. Similarly, the lower the hydrogen ion concentration, the higher the pH value, and the solution is more alkaline.
Hence, the pH value of the solutions with the given [H+] values can be calculated as follows:
a) [H+] = 0.01pH
= -log[H+]
= -log(0.01)
= 2.0
b) [H+] = 1.0 x 10 –8 pH
= -log[H+]
= -log(1.0 x 10 –8)
= 8.0
c) [H+] = 4.67 x 10 –4 pH
= -log[H+]
= -log(4.67 x 10 –4)
= 3.33
It is important to remember that the hydrogen ion concentration [H+] is measured in moles per liter (mol/L), and the pH value of a solution can be calculated using the formula: pH = -log[H+].
Also, the negative sign in the formula indicates that the pH value is inversely proportional to the hydrogen ion concentration.
To know more about H+ visit:
https://brainly.com/question/11171476
#SPJ11
Determine the pH of a 0.35 M aqueous solution of CH3NH2 (methylamine). The Kb of methylamine is 4.4 × 10−4.
can you please show your work and explain the steps on how you get 12.09 as the answer
Answers
The Kb of methylamine is 4.4 × 10⁻⁴.
The pH of a 0.35 M aqueous solution of CH₃NH₂ (methylamine) can be determined using the Henderson-Hasselbalch equation. The Henderson-Hasselbalch equation states that the pH of a solution can be determined by taking the negative logarithm of the base-to-acid ratio.
In this case, the base-to-acid ratio is equal to the concentration of the base, CH₃NH₂, divided by the acid, CH₃NH³+. The acid dissociation constant, Kb, is then used to calculate the concentration of the acid. The Kb of methylamine is 4.4 × 10⁻⁴.
After plugging in the appropriate values into the Henderson-Hasselbalch equation, the pH of the solution can be calculated to be 12.09. This indicates that the solution is basic in nature, as all pH values greater than 7 are considered to be basic.
Know more about Henderson-Hasselbalch here
https://brainly.com/question/13423434#
#SPJ11
(GIVING BRAINLIEST)
Balance each of the following chemical equations below

Answers
Explanation:
A.
AgNO₃ + KCl → AgCl + KNO₃ (Already Balanced)
B.
H₂O + SO₃ → H₂SO₄ (Already balanced)
C.
2KI + Cl₂ → 2KCl + I₂
D.
2NaHCO₃ → Na₂CO₃ + H₂O + CO₂
E.
Zn + 2HCl → ZnCl₂ + H₂
F.
BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl
G.
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
H.
2Al + 3CuCl₂ → 2AlCl₃ + 3Cu
To separate a mixture of large gravel and sand, the most effective method would be
Answers
Answer:
Sieve
Explanation:
A sieve or screen would be the best tool to use when separating a mixture of sand and gravel.
Answer:
the third option on edge:)
Explanation:
Question Megan made a model of the respiratory system using a water bottle and balloons. When she pulled down on the balloon stretched across the bottom of the bottle, the balloon hanging inside the bottle inflated. What organ does the balloon hanging inside the bottle represent?
Answers
Answer:
The lung
Explanation:
The model of the respiratory system made by Megan consists of two balloons. The first balloon stretched across the bottom of the bottle represents the diaphragm which contracts and relaxes to allow air in and out of the lungs. The balloon inside the bottle represents one lung.
Breathing in causes the balloon inside the bottle to be filled with air. This is preceded by the expansion of the diaphragm which makes the lungs to be filled with air. Breathing out causes a contraction of the diaphragm thus making the lungs to let out air.
Answer:
You're Lungs
Explanation:
When you breathe in, air flows into your lungs. When you breathe out, air flows out of your lungs. The balloon inside the bottle is like one of your lungs.
) which has the most electron pairs (both shared and unshared) around the central atom? a b c d (b) which has the most unshared pairs around the central atom? a b c d (c) do any have only shared pairs around the central atom? a b c d none
Answers
Considering the molecular shapes:
a.) The molecular shape with the most electron pairs around the central atom is option A.
b.) The shape with the most unshared pairs around the central atom is option A.
c.) The one with only shared pairs around the central atom is option C and D
How are shared and unshared electrons determined?Shared pairs of electrons represented as bonding pairs while unshared pairs of electrons are pairs that are not bonded also known as lone pairs.
These electrons are determined by the total number of bonding and un-bonding electrons of a compound using the equation, "S = N - A, where S is the total number of shared electrons, N the number of valence shells while A is the number of electrons available in valence shells of atomic structure.
Full question is:
a) O; (b) He; (c) F; (d) H; (e) P
Consider the following molecular shapes.
(a) Which has the most electron pairs (both shared and unshared) around the central atom?
(b) Which has the most unshared pairs around the central atom?
(c) Do any have only shared pairs around the central atom?
(a) A
(b)A
(c)C&D
Learn more on electrons here: https://brainly.com/question/22238351
#SPJ1
Water is a polar molecule. What does that mean?
A. It is a molecule with too many protons
B. it is a molecule with identical charges on opposite ends
C. it is a molecule with no charge
D. it is a molecule with opposite charges on opposite ends
Answers
a 100.0 ml solution of naoh reaches the equivalence point when 30.22 ml of a 0.0750 m solution of hcl is added from the burette.
Answers
The answer is 0.0026.
Solution:
(V₁) Volume of NaOH = 100 mL
(V₂) Volume of HCl = 30.22 mL
(M₂) molarity of HC = 0.0750 m
(m₁) molarity of NaOH =
By using relation
M₁V₁ = M₂V₂
M₁ = M₂ V₂/V₁
M₁ = 0.0750ML*30.22mL/100 mL
M₁ = 0.0226
Now
molarity of NaOH = mole of NaOH/Volume of NaOH (in L)
mole of NaOH = molarity x volume
= 0.0226 mol/L X 100 X 10⁻³ L
= 0.0026 mol So the mole of NaOH is 0.0026.
When titrating NaOH with HCl using methyl orange as an indicator the endpoint color changes from yellow to red, so add HCl to the burette. This is just for ease of calculation. Put the hydrochloric acid in the burette and the soda solution in the Erlenmeyer flask. Methyl orange is used as an indicator.
Learn more about The equivalence point here:- https://brainly.com/question/2496608
#SPJ4
2N2 + 502 --> 2N205
Based on the above reaction, if 7 moles of Ny react completely, how many moles of Oz must also react?
ASAP LOOK AT PICTURE

Answers
Answer:
wait are you talking about the bottom question??
PLEASE HELP!! What is the pH of a solution with a [H3O+] concentration of 3.4 x 10-11 M? Is the solution acidic, basic, or neutral?
Answers
\(\\ \tt\leadsto pH=-log[H^+][/texM
\(\\ \tt\leadsto pH=-log[3.4\times 10^{-11}]\)
\(\\ \tt\leadsto pH=-log3.4-log10^{-11}\)
\(\\ \tt\leadsto pH=-0.53+11\)
\(\\ \tt\leadsto pH=10.47\)
Basic
Considering the definition of pH, the pH of the solution is 10.47 and since it is higher than 7, it is a basic substance.
Definition of pHpH is the Hydrogen Potential. It is a measure of acidity or alkalinity that indicates the amount of hydrogen ions present in a solution or substance.
Mathematically, pH is calculated as the negative base 10 logarithm of the activity of hydrogen ions:
pH= - log [H⁺]= - log [H₃O⁺]
Numerical scale of pHThe numerical scale that measures the pH of substances includes the numbers from 0 to 14. The pH value 7 corresponds to neutral substances. Acidic substances are those with a pH lower than 7, while basic substances have a pH higher than 7.
pH in this caseIn this case, you know [H₃O⁺]= 3.4×10⁻¹¹ M.
Replacing in the definition of pH:
pH= -log (3.4×10⁻¹¹ M)
Solving:
pH= 10.47
Finally, the pH of the solution is 10.47 and since it is higher than 7, it is a basic substance.
Learn more about pH:
brainly.com/question/3992824
#SPJ1
the reaction of methyl iodide with sodium azide, nan3, proceeds by an sn2 mechanism. what is the effect of doubling the concentration of nan3 on the rate of the reaction? a. the rate remains the same b. the rate decreases by a factor of 2 c. the rate increases by a factor of 2 d. the rate increases by a factor of 4
Answers
The reaction of methyl iodide with sodium azide, NaN₃, proceeds by an SN2 mechanism. The effect of doubling the concentration of NaN₃ on the rate of the reaction is the rate increases by a factor of 2. The correct answer is C.
The alkyl halide is attacked by the nucleophile as the SN2 mechanism. as a partial bond is formed involving the nucleophile and carbon atom. Halide and carbon's bond partially dissolves. A transition is created where there are five bonds around the carbon atom. The product is created when the leaving group, which is a halide, removes the electrons from the C-X link and departs the molecule.
R-X + Nu⁻ → Nu
--R--X → Nu-R + X⁻
Since a transition state that contains both a nucleophile and a substrate is used for the nucleophilic substitution. It was discovered that the concentration of the substrate and the nucleophile both affect the rate of the reaction.
Rate = k [substrate] [nucleophile]
Since sodium azide is the nucleophilic species and methyl iodide is the substrate,
Rate = k[CH₃I][NaN₃]
both the reactants take part in the reaction and the rate law for the reaction. As a concentration of NaN₃ is doubled, the new rate becomes,
Rate' = k[CH₃I][2 NaN₃]
= 2 k[CH₃I][NaN₃]
= 2 Rate
Learn more about SN2 mechanism at https://brainly.com/question/29561608
#SPJ4
What is the flow rate? How is it measured ?
Answers
how much is 3.5 gallons in cups
Answers
Answer:
3.6 gallons are equal to 56 cups
The four nitrogen bases are thymine, cytosine, guanine, and adenine. True or false
Answers
Answer:
True
Explanation:
a chemist wanted to make a buffer solution and used of of a concentration, and mixed it with of a solution of ?
Answers
To make a buffer solution, you must use solutions of a weak acid and its conjugate base.
For example, if you wanted to make a buffer solution with a pH of 4.5, you would mix a solution of acetic acid (CH3COOH) and sodium acetate (CH3COONa).
The acetic acid is the weak acid and the sodium acetate is its conjugate base. The concentrations of the two solutions should be in a ratio of 1:1 in order to maintain the desired pH.
To know more about buffer solution:
https://brainly.com/question/30737303
#SPJ11
What is the mass of an atom of krypton? explain
Answers
Answer:
Krypton. Atomic Mass. 83.80u. Electron Configuration. [Ar]4s23d104p6.
Atomic Mass: 83.80u
Electron Configuration: 4s23d104p6
Year Discovered: 1898
How many liters of water are needed to prepare a 1.67M solution of Ba(OH)2 if you need to dissolve 235g of it?
Answers
Answer: 2.04
Explanation:
Answer:
Approximately \(0.821\; \rm mol\).
Explanation:
Look up the relative atomic mass of \(\rm Ba\), \(\rm O\), and \(\rm H\) on a modern periodic table:
\(\rm Ba\): \(137.327\).\(\rm O\): \(15.999\).\(\rm H\): \(1.008\).Calculate the formula mass of \({\rm Ba(OH)_2}\):
\(\begin{aligned}& M({\rm Ba(OH)_2}) \\ &= 137.327 + 2\times(15.999 + 1.008) \\ &\approx 171.334\; \rm g \cdot mol^{-1}\end{aligned}\).
Calculate the number of moles of \({\rm Ba(OH)_2}\) formula units in that \(235\; \rm g\) of this compound:
\(\begin{aligned}& n({\rm Ba(OH)_2}) \\ &= \frac{m({\rm Ba(OH)_2})}{M({\rm Ba(OH)_2})} \\ &= \frac{235\; \rm g}{171.334\; \rm g \cdot mol^{-1}} \approx 1.37159\; \rm mol \end{aligned}\).
Calculate the volume of a \(c({\rm Ba(OH)_2}) = 1.67\; \rm mol \cdot L^{-1}\) with approximately \(n({\rm Ba(OH)_2}) = 1.37159\; \rm mol\) of the solute:
\(\begin{aligned}& V({\rm Ba(OH)_2}) \\ &= \frac{n({\rm Ba(OH)_2})}{c({\rm Ba(OH)_2})} \\ &= \frac{1.37159\; \rm mol}{1.67\; \rm mol \cdot L^{-1}} \approx 0.821\; \rm L \end{aligned}\).
Based on the chemical equation, use the drop-down menu to choose the coefficients that will balance the chemical
equation:
(( H20-( H2+( 02
Answers
Answer:
\(2H_2O\rightarrow 2H_2+O_2\)
Explanation:
Hello there!
In this case, for the reaction by which water is decomposed to molecular hydrogen and oxygen:
\(H_2O\rightarrow H_2+O_2\)
It is necessary to perform the inspection balance process since there is a dissimilar number of atoms of oxygen on both sides; therefore, by putting a 2 on water we balance oxygen:
\(2H_2O\rightarrow H_2+O_2\)
But now, there are four hydrogens on the left; therefore, we put a 2 on hydrogen to finally balance it:
\(2H_2O\rightarrow 2H_2+O_2\)
And obviously, the coefficient in oxygen is an unwritten 1.
Best regards!
Which of the following elements has the lowest electronegativity?
A. Barium
B. Magnesium
C. Strontium
D. Calcium
Answers
Answer:
A. Barium
Explanation:
hope this helps! :)
The first scale of electronegativity was developed by Linus Pauling and on his scale barium has a value of 0.89 on a scale running from from about 0.7 (an estimate for francium) to 2.20 (for hydrogen) to 3.98 (fluorine).
Describe the strategy concerning the energy conservation (energy
saving) of chemical process, by using the keywords: exergy; energy
harvesting; power generation; thermoelectric conversion; insulator;
Answers
The chemical process involves the conversion of raw materials into usable products. It uses a significant amount of energy, which results in high operating costs.
Therefore, energy conservation is a crucial factor in the chemical industry, which would help reduce the operating cost and environmental pollution. Several strategies have been developed, which include exergy, energy harvesting, power generation, thermoelectric conversion, and insulation.Exergy:Exergy is a measure of the energy that is available to do work. It is the portion of the energy that can be converted to useful work.
Therefore, exergy analysis is used to evaluate the efficiency of the chemical process and identify areas where energy can be saved.
To know more about raw materials visit:
https://brainly.com/question/30506076
#SPJ11
Which are steps of mitosis? Select 4 options.
prophase
metaphase
interphase
anaphase
telophase
Answers
Metaphase
Anaphase
Telophase
Answer:
Prophase, metaphase, anaphase, telophase
Explanation:
What is the importance of the fundamental laws of chemistry?
Answers
Law of Conservation of mass: Mass cannot be created nor destroyed. Reference: Antoine Lavoisier – By carefully weighing the reactants & products of chemical reactions. The laws of chemical combination describe the basic principles obeyed by interacting atoms and molecules, interactions that can include many different combinations that happen in many different ways. This amazing diversity of interactions allows for an astounding variety of chemical reactions and compounds. The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reaction. Modern chemistry is based on several fundamental laws, including:
The law of multiple proportions.The law of definite proportions.The law of conservation of mass.