A hydorcarbon cxhy has mass ratio between hydorgen and carbon 1:10. 5. One litre of the hydrogen at 127c and 1 atm pressure weighs 2. 8 g,find the molecular formula of the hydrocarbon
Answers
Rounded to the nearest whole number, y is 42. Therefore, the molecular formula of the hydrocarbon is C4H42.
To find the molecular formula of the hydrocarbon, we first need to determine the molecular weight. We know that the mass ratio between hydrogen and carbon is 1:10, which means that for every 1 gram of hydrogen, there are 10 grams of carbon in the molecule.
Let's assume that we have x number of carbon atoms and y number of hydrogen atoms in the molecule. The molecular weight can then be expressed as:
Molecular weight = (x x atomic weight of carbon) + (y x atomic weight of hydrogen)
Since the mass ratio between hydrogen and carbon is 1:10, we can write:
y = 10x
Now, we can substitute y in the equation for molecular weight:
Molecular weight = (x x atomic weight of carbon) + (10x x atomic weight of hydrogen)
Molecular weight = x(atomic weight of carbon + 10 x atomic weight of hydrogen)
We also know that one liter of hydrogen at 127°C and 1 atm pressure weighs 2.8 g. Using the ideal gas law, we can calculate the number of moles of hydrogen in one liter:
PV = nRT
n = PV/RT
n = (1 atm x 1 L) / (0.0821 L.atm/mol.K x 400 K)
n = 0.0305 mol
The molecular weight of the hydrocarbon can be calculated as follows:
Molecular weight = 2.8 g / 0.0305 mol
Molecular weight = 91.80 g/mol
Now, we can solve for x in the equation for molecular weight:
91.80 g/mol = x(12.01 g/mol + 10 x 1.01 g/mol)
91.80 g/mol = 12.01x + 10.10x
91.80 g/mol = 22.11x
x = 4.15
Since x represents the number of carbon atoms in the molecule, we can round it to the nearest whole number, which is 4. Similarly, y can be calculated as:
y = 10x = 41.5
Rounded to the nearest whole number, y is 42. Therefore, the molecular formula of the hydrocarbon is C4H42.
To know more about molecular formula, visit:
https://brainly.com/question/28647690#
#SPJ11
Related Questions
an unknown solution was determined to be basic. when this solution was combined with lemon juice (a known acid) no visible change occurred. this means that the unknown could be
Answers
The unknown solution could be a variety of basic substances, such as sodium hydroxide, potassium hydroxide, or calcium hydroxide, among others. Further testing and analysis would be needed to determine the exact nature of the unknown solution.
What is Solution?
In chemistry, a solution is a homogeneous mixture composed of two or more substances. The substance present in the largest amount is called the solvent, while the other substances present in smaller amounts are called solutes. The solutes are dissolved in the solvent to form a homogeneous mixture.
When an acid and a base are combined, they can undergo a neutralization reaction that results in the formation of a salt and water. If the acid is added to a basic solution, the resulting mixture will typically have a pH that is closer to neutral than either the acid or the base on their own.
Learn more about Solution
https://brainly.com/question/25326161
#SPJ1
Which refers to radiant energy that comes from the Sun and interacts with surfaces or objects near or on Earth?
O solar radiation
O greenhouse energy
O solar reflection
O energy budget
Answers
Answer:
the answer is a
Explanation:
solar radiation
the molecular formula mass for sulfur dioxide is 54 grams. T and f
Answers
The statement that the molecular formula mass for sulfur dioxide is 54 grams is false. The correct molecular formula mass for sulfur dioxide is 64 grams.
Sulfur dioxide (SO2) is a chemical compound composed of one sulfur atom (atomic mass of 32 grams) and two oxygen atoms (atomic mass of 16 grams each). To calculate the molecular formula mass of sulfur dioxide, we add the atomic masses of each atom in the compound. In this case, 32 grams (sulfur) + 16 grams (oxygen) + 16 grams (oxygen) equals a total molecular formula mass of 64 grams. Therefore, the molecular formula mass for sulfur dioxide is 64 grams, not 54 grams.
Learn more about formula mass here: brainly.com/question/28647347
#SPJ11
1. Draw the curved mechanism arrows that show the deprotonation of phenol by NaOH and draw the major organic product
2. Draw the curved mechanism arrows that show the sn2 reaction of phenoxide with chloroethane and draw the major organic product
Answers
Deprotonation of phenol by NaOHPhenol is a weak acid with a pKa value of 10, so it is not very acidic but it can be deprotonated with a strong base like sodium hydroxide (NaOH). The mechanism for this reaction is a simple acid-base reaction where NaOH acts as a base and removes the proton (H+) from phenol.
The curved arrow mechanism is shown below:Here, the Na+ ion comes in and removes the H+ ion from the O-H bond in phenol and forms water as a by-product. The major organic product is phenoxide ion (C6H5O−) which is formed after the deprotonation reaction.SN2 reaction of phenoxide with chloroethane Phenoxide ion is a good nucleophile because it has a negative charge on the oxygen atom which is able to attack the positively charged carbon atom in chloroethane. The mechanism for this reaction is a nucleophilic substitution (SN2) reaction where the phenoxide ion acts as a nucleophile and replaces the chloride ion in chloroethane. The curved arrow mechanism is shown below:Here, the phenoxide ion attacks the carbon atom in chloroethane, and the chloride ion leaves as a leaving group. The major organic product is ethyl phenyl ether (C6H5OC2H5) which is formed after the SN2 reaction.
For more information on Deprotonation visit:
brainly.com/question/30706409
#SPJ11
What environmental factors affect animal characteristics?
factor that could affect

Answers
Answers:
There's many factors, such as the temperature, rainfall, food available, and the surrounding terrain, that all can effect an animal's characteristics.
The three properties of objects are:
Answers
Answer:
they have shape.
they have volume.
they occupy space.
answer asap pls i give brainliest

Answers
Answer:
B. Revolution is a planet's movement around the sun causing four seasons the occur. Rotation is the spinning of the planet on its axis causing day and night.
What are some example of chemistry outside ?
Answers
Photosynthesis
Cell Respiration
Kf crystallizes in a face-centered cubic cell. what is the total number of ions (k ions and f - ions) that lie within a unit cell of kf?
Answers
Within a unit cell of KF, there are a total of 8 K⁺ ions + 6 F⁻ ions = 14 ions (K⁺ and F⁻) in total.
In crystallography, a unit cell is the basic repeating structural unit of a crystal lattice. It is a three-dimensional parallelepiped that represents the smallest repeating pattern of the crystal structure. The unit cell is used to describe the arrangement of atoms, ions, or molecules within a crystal.
A unit cell is defined by its three edges, which determine its dimensions, as well as the angles between these edges. There are several types of unit cells, including cubic, tetragonal, orthorhombic, monoclinic, triclinic, and hexagonal, each characterized by different edge lengths and angles.
In a face-centered cubic (FCC) unit cell, there are four ions located at each of the eight corners and one ion at the center of each face. Since KF is composed of one potassium ion (K+) and one fluoride ion (F-), we can determine the total number of ions within the unit cell.
The potassium ions (K⁺) are present only at the corners, so there are 8 corners x 1 K⁺ ion = 8 K⁺ ions.
The fluoride ions (F⁻) are present at the center of each face, so there are 6 faces x 1 F⁻ ion = 6 F⁻ ions.
Learn more about Unit cell, here:
https://brainly.com/question/13110055
#SPJ4
you are to prepare 60 grams of a 3% progesterone cream. you have progesterone 1% and progesterone 10% cream in stock. how many grams of each cream will be needed?
Answers
Let x be the amount of 1% progesterone cream needed, and y be the amount of 10% progesterone cream needed to prepare 60 grams of 3% progesterone cream.
We can set up a system of equations based on the information given:
x + y = 60 (total cream needed)
0.01x + 0.1y = 0.03(60) (total progesterone needed)
Simplifying the second equation:
0.01x + 0.1y = 1.8
Multiplying both sides by 100 to get rid of the decimal:
x + 10y = 180
Now we have two equations:
x + y = 60
x + 10y = 180
Subtracting the first equation from the second, we get:
9y = 120
Dividing both sides by 9:
y = 13.33
Substituting y back into the first equation:
x + 13.33 = 60
Subtracting 13.33 from both sides:
x = 46.67
Therefore, we need 46.67 grams of 1% progesterone cream and 13.33 grams of 10% progesterone cream to prepare 60 grams of 3% progesterone cream.
What is a cream ?In general, a cream refers to a semi-solid or liquid emulsion that is made by mixing oil and water with other ingredients such as emulsifiers, thickeners, and stabilizers.
To know more about cream visit :
https://brainly.com/question/31063425
#SPJ1
A sample of nitrogen gas is collected over water at temperature of 20.0˚C. What is the pressure of the nitrogen gas if atmospheric pressure is 1.01atm? (vapor pressure of water at 20.0˚C is 17.5 torr)
Answers
Answer:
\(P_N=0.987atm\)
Explanation:
Hello there!
In this case, for these problems about collecting a gas over water, we must keep in mind that once the gas has been collected, the total pressure of the system is given by the atmospheric pressure, in this case 1.01 atm. Next, since we also have water in the mixture, we can write the following equation:
\(P_T=P_w+P_N\)
Thus, by solving for the pressure of nitrogen and using consistent units, we obtain:
\(1.01atm=17.5torr*\frac{1atm}{760torr} +P_N\\\\P_N=1.01atm-0.023atm\\\\P_N=0.987atm\)
Zn + Cu SO 4 —>
What’s the reaction
Answers
Answer:
Single displacement reaction
Explanation:
You have an element (Zinc) + a compound that consists of an element (Copper) and a polyatomic ion (Sulfate)
Since there's one element and one compound, the metals in both trade places, giving us Zinc Sulfate and Copper as our products.
This should be the complete balanced equation:
Zn + CuSO4 -------> ZnSO4 + Cu
The equation for the saturated solution equilibrium of potassium nitrate (KNO3 ) is shown below.
KNO (s) + energy K+ (aq) + NO – (aq) 33
Compare the rate of dissolving KNO3 with the rate of recrystallization of KNO3 for the saturated solution.
Answers
Answer:
In a saturated solution of potassium nitrate (KNO3), the rate of dissolution and the rate of recrystallization are equal. This equilibrium state is reached when the amount of KNO3 that dissolves equals the amount that recrystallizes from the solution.
The equilibrium can be influenced by various factors such as temperature and pressure. For example, if you increase the temperature of the solution, the solubility of potassium nitrate increases, meaning that more KNO3 can dissolve before reaching saturation. This would momentarily increase the rate of dissolution until a new equilibrium is reached where the rates of dissolution and recrystallization are equal again, but at a higher concentration of KNO3.
To summarize, in a saturated solution of KNO3, the rate of dissolving KNO3 is equal to the rate of recrystallization of KNO3. This is a characteristic of dynamic equilibrium in solutions.
Rank 1M of these compounds in order of increasing hydrogen ion concentration: weak acid, strong acid, strong base, weak base.
Answers
The compounds ranked in order of increasing hydrogen ion concentration are: strong base, weak base, weak acid, strong acid.
In order to determine the order of increasing hydrogen ion concentration, we need to consider the strengths of the acids and bases. Acids donate hydrogen ions (H+) to a solution while bases accept hydrogen ions. Strong acids and bases completely dissociate in water to form H+ or OH- ions, respectively. Weak acids and bases only partially dissociate, resulting in fewer H+ or OH- ions in solution. Thus, the concentration of H+ ions will be higher in solutions containing strong acids and lower in solutions containing weak acids or bases.
Ranking the compounds in order of increasing hydrogen ion concentration:
1. Strong base
2. Weak base
3. Weak acid
4. Strong acid
The strong base will accept H+ ions, resulting in a lower concentration of H+ in the solution. The weak base will accept some H+ ions but not as many as the strong base, resulting in a slightly higher concentration of H+. The weak acid will donate some H+ ions but not as many as the strong acid, resulting in a higher concentration of H+. The strong acid will donate many H+ ions, resulting in the highest concentration of H+.
Therefore, the correct ranking of the compounds in order of increasing hydrogen ion concentration is: strong base, weak base, weak acid, strong acid.
To know more about hydrogen ion concentration, refer to the link below:
https://brainly.com/question/17571892#
#SPJ11
Write a play that explains the reproduction of flowers and plants. You can make this a comedy, a tragedy, or just a fact-filled play. Include some of the plant parts and the importance of pollination in the play. Possible characters could include Fred the Flower, Bratty Bee, Whistling Wind, etc. You can include friends and family members, or do a solo act and play all the parts yourself. Have fun with it!
Once you have completed your assignment, turn it in to your teacher. To see how your assignment will be graded, click the "View Assignment" link.
please help..
Answers
Answer:
photosynthesis
Explanation:
Pleasee help me :(
If a solution of aspirin has a [H3O+] = 1.7 x10 -3 M, what is the pH of the solution?
Answers
Answer:
2.77Explanation:
pH is defined as the negative logarithm of the hydrogen ion concentration of a substance.
In order to find the pH we use the formula;
\( \bold{pH = -log([{H_3O}^{+}])} \)
From the question
\( [{H_3O}^{+}]\) = \( {1.7 \times 10}^{-3} \: M \)
We have
\( pH = -log({1.7 \times 10}^{-3}) \\ = 2.769555 \)
We have the final answer as
pH = 2.77BARINLY PLS HELP ME ASP !!! it’s science I need help fast pls

Answers
Answer:
Its D. All of the above
ibuprofen c13h18o2 that is manufactured in michigan contains 75.69% by mass carbon, 8.80% hydrogen, and 15.51% oxygen. if you buy some ibuprofen for a headache while you are on vacation in germany, how do you know that it has the same percentage composition as the ibuprofen you buy at home?
Answers
Law of definite composition: As long as something has the same formula, its mass will be the same.
According to the Law of Definite Proportions, a chemical compound will always include the same weight proportions of each element, regardless of the quantity or source. For instance, 21.5 grammes of carbon and 28.5 grammes of oxygen make up a 50-gram sample of carbon monoxide.
What is Ibuprofen ?Ibuprofen is a medication used to treat pain, fever, and inflammation, and is a member of the nonsteroidal anti-inflammatory drug (NSAID) family. This includes menstruation pain, migraines, and rheumatoid arthritis. The patent ductus arteriosus of a premature newborn can also be sealed with this technique.
Ibuprofen is a painkiller that can be bought over-the-counter without a prescription. It is a member of the group of drugs referred to as non-steroidal anti-inflammatory drugs.Learn more about Ibuprofen here:
https://brainly.com/question/13450929
#SPJ4
With what volume of 5. 00 MHF will 4. 03 g of calcium hydroxide react completely, according to
the following reaction?
2HF + Ca(OH)₂ →CaF₂ + 2H₂O
Answers
The balanced chemical equation for the reaction between hydrofluoric acid (HF) and calcium hydroxide (Ca(OH)2) is:
2HF + Ca(OH)2 → CaF2 + 2H2O
To determine the volume of 5.00 M HF needed to react completely with 4.03 g of Ca(OH)2, we need to use stoichiometry.
First, we need to determine the number of moles of Ca(OH)2: molar mass of Ca(OH)2 = 74.09 g/mol number of moles of Ca(OH)2 = mass / molar mass = 4.03 g / 74.09 g/mol = 0.0544 mol
From the balanced chemical equation, we see that 2 moles of HF react with 1 mole of Ca(OH)2. Therefore, the number of moles of HF required is: moles of HF = 2 x moles of Ca(OH)2 = 2 x 0.0544 mol = 0.1088 mol
Finally, we can calculate the volume of 5.00 M HF needed using the following equation:
moles = concentration x volume
volume = moles / concentration = 0.1088 mol / 5.00 mol/L = 0.0218 L = 21.8 mL
Learn more about chemical equation here:
brainly.com/question/32166289
#SPJ11
Increasing which factor will cause the gravitational force between two objects to decrease?
weights of the objects
distance between the objects
acceleration of the objects
masses of the objects
Answers
Answer:
mass increase!
Explanation:
<3
Answer:
b.distance between the objects
Explanation:
If you pull objects apart from each other they don't have as strong of a gravitational pull. think of it like a magnet the heavier the magnets the more power they can hold, the bigger they are the more surface area they have, and when you pull 2 magnets apart they slowly come weaker to each other.
The concentration of TCE is 28ppm, convert this to molarity. The molecular weight of TCE is 132g/mole.
Answers
The concentration of TCE is 28ppm, convert this to molarity. The molecular weight of TCE is 132g/mole. The molarity of TCE is, therefore, 0.000212 M.
The term ppm stands for parts per million. This term is used to describe the concentration of a substance present in a given solution or mixture. To find the molarity of TCE, the given concentration of TCE in ppm needs to be converted into molarity.
The given concentration of TCE is 28ppm.
1 ppm = 1 mg/L1mg/L = 10^-3 g/L
The molecular weight of TCE is 132g/mole.
Conversion of ppm to g/L is given as follows:
28 ppm = 28 mg/L
=28 x 10^-3 g/L
Number of moles of TCE in 1 L is:
mass of TCE/molecular weight of TCE = 28 x 10^-3/132
= 0.000212 mole/L.
The molarity of TCE is, therefore, 0.000212 M.
To know more about molarity visit:
https://brainly.com/question/31545539
#SPJ11
What is the frequency of radiation whose wavelength is 2.40×10
Answers
Answer:
24
Explanation:
2.40*10=24.0
Which part of the mouth carries out each function? breaks down carbohydrates into sugars: cuts up food into smaller pieces:
Answers
Answer:
Saliva and teeth in this order
Explanation:
Make me BRANLIEST please
I need help NOWW PLEASE HELP ME




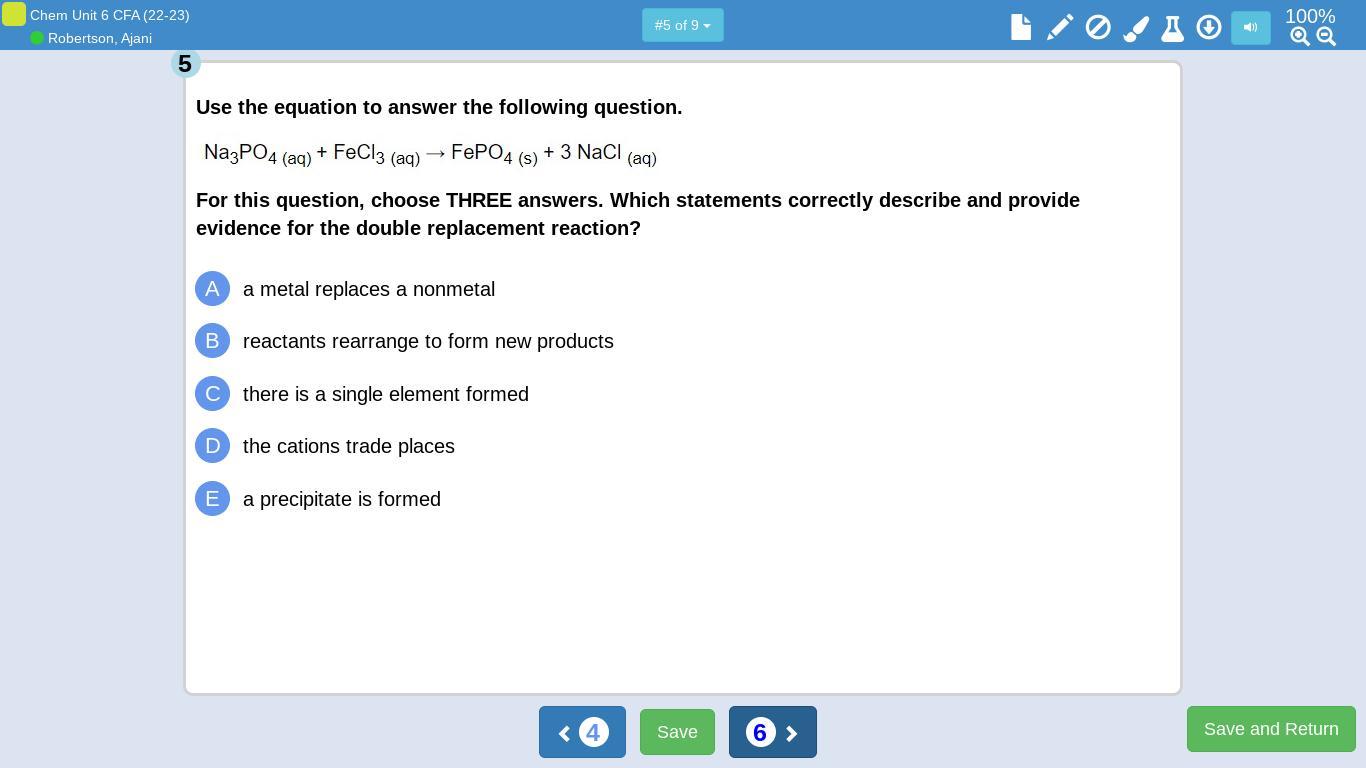
Answers
The reaction of Al₂S₃ with Li forms the displaced product Li₂S₃ and Al. The correct formula of the compound with X having one valence electron and Z with 6 valence electrons is XZ₂. The given reaction in the third image is a single replacement reaction and the reaction in the fourth image is double displacement. Options A, B and E are correct for the reaction in fifth image.
What is displacement reaction?In displacement reactions , an atom or group is displaced by other atom or group from a reactant. In double displacement reactions, two group or atoms are displaced between two reactants.
In the reaction between Al₂S₃ with Li forms the displaced product Li₂S₃ and Al. It is a single displacement reaction.
The element X with 1 valence electron will form a compound with Z having 6 valence electrons with the formula XZ₂. Z needs two electrons to attain octet. Thus two X provides one electron by each to Z.
The reaction of Na metal with HCl producing NaCl and hydrogen gas is a single displacement reaction. Whereas, the reaction between NaCl and MgF2 is double displacement because, both Cl and F atoms are displacing their position.
Sodium phosphate reacts with ferric chloride giving the ferric phosphate precipitate. Here the metals replaces nonmetals and the reaction involves formation of a precipitate. Thus, option A, B and E are correct.
To find more on displacement reaction, refer here:
https://brainly.com/question/3172917
#SPJ1
What is the ATP for weeks 1 to 8?
Consider this MPS and the ATP calculations for a firm
Answers
The ATP (Available-to-Promise) for weeks 1 to 8 is the amount of inventory that a firm can commit to fulfilling customer orders within that time frame.
To calculate the ATP, you need to consider the MPS (Master Production Schedule) and the ATP calculations.
1. Start by looking at the MPS for weeks 1 to 8. The MPS represents the planned production quantities for each week.
2. Determine the beginning inventory for week 1. This is the inventory available at the start of week 1.
3. Add the MPS quantity for week 1 to the beginning inventory. This gives you the available inventory for week 1.
4. Subtract customer orders for week 1 from the available inventory. This gives you the remaining inventory after fulfilling customer orders for week 1.
5. Repeat steps 3 and 4 for each subsequent week, using the previous week's remaining inventory as the beginning inventory for the next week.
6. Continue this process until you reach week 8, calculating the available inventory and subtracting customer orders for each week.
7. The resulting values represent the ATP for weeks 1 to 8.
The ATP can fluctuate as new customer orders are received or changes are made to the MPS. Regular monitoring and adjustment of the ATP is necessary to ensure accurate order fulfillment.
Learn more about inventory from:
https://brainly.com/question/26977216
#SPJ11
PLEASE HELP ASAP:
Two objects are placed a certain distance from each other. The amount of gravitational force between the two objects depends on their masses and the distance between them. As the masses of the objects decrease, the force of gravity between them _____________. As the distance between the objects decreases, the force of gravity between them ______________.
Answers
Two objects are placed a certain distance from each other. The amount of gravitational force between the two objects depends on their masses and the distance between them. As the masses of the objects decrease, the force of gravity between them increases. As the distance between the objects decreases, the force of gravity between them decreases.
What is gravitational force?Gravitational force is defined as the force of attraction that a body of mass M, exerts on another body that is found within its region of space that this force acts.
The law of gravitational attraction states that the gravitational force of attraction between two bodies of masses m₁ and m₂ is directly proportional to the product of their asses and inversely proportional to the square of the distance between them, r.
Learn more about gravitational force at: https://brainly.com/question/862529
#SPJ1
what will happen when iodine solution is added to
potassium chloride.
Answers
Answer:
The chlorine is more reactive than the iodine in potassium iodide. This causes the iodine to be displaced from the compound and chloride ions take its place instead.
Explanation:
Answer:
Potassium iodide + chlorine gas. In this reaction when potassium iodide solution is put into chlorine gas a rapid reaction occurs and black iodine solid is formed along with potassium chloride solution. The chlorine has kicked the iodide out.
Explanation:
what would be the initial rate of formation of br2
Answers
The initial rate of formation of Br2 can be determined by examining the rate law for the reaction in which it is formed.
The rate law is an equation that relates the rate of a reaction to the concentrations of the reactants and the rate constant.
For example, if the reaction is:
2Br- + Cl2 → 2Cl- + Br2
The rate law would be:
rate = k[Br-][Cl2]
To find the initial rate of formation of Br2, you would need to know the initial concentrations of the reactants (Br- and Cl2) and the rate constant (k). Once you have these values, you can plug them into the rate law equation and solve for the initial rate.
For example, if the initial concentrations of Br- and Cl2 are both 0.1 M and the rate constant is 2.5 x 10^-3 M^-1s^-1, the initial rate of formation of Br2 would be:
rate = (2.5 x 10^-3 M^-1s^-1)(0.1 M)(0.1 M) = 2.5 x 10^-5 M/s
So the initial rate of formation of Br2 in this example would be 2.5 x 10^-5 M/s.
To know more about initial rate of formation refer here:
https://brainly.com/question/2860031#
#SPJ11
*
The prevailing weather patterns of a region
Answers
Answer:
noun. the composite or generally prevailing weather conditions of a region, as temperature, air pressure, humidity, precipitation, sunshine, cloudiness, and winds, throughout the year, averaged over a series of years. a region or area characterized by a given climate: to move to a warm climate.
Explanation:
explain what you know about alchemy
Answers
Alchemy is an ancient practice that is considered to be the precursor to modern chemistry. It was primarily concerned with the transmutation of base metals into gold, the creation of a universal solvent or elixir of life, and the discovery of a universal cure for diseases. Alchemists believed that these goals could be achieved through a combination of chemical processes and spiritual practices.
One of the key concepts in alchemy is the idea of the "philosopher's stone," a substance that was believed to have the ability to turn base metals into gold and grant eternal life. Alchemists spent centuries searching for the philosopher's stone, experimenting with various substances and processes in an attempt to create it.
Although alchemy is often considered to be a pseudoscience, it did contribute to the development of modern chemistry. Many of the techniques and apparatus used by alchemists, such as distillation and the use of laboratory glassware, are still used in modern chemistry labs.
Additionally, alchemists were among the first to discover and isolate many chemical elements, such as phosphorus and mercury.
To know more about Alchemy here:
https://brainly.com/question/5997100#
#SPJ11