Answers
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
The volume of the gas at 30 °C is approximately 760.67 mL.
To determine the volume of the gas at 30 °C, we can use the combined gas law equation, which relates the initial and final conditions of temperature and volume for a gas.
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 and P2 are the initial and final pressures, respectively
V1 and V2 are the initial and final volumes, respectively
T1 and T2 are the initial and final temperatures in Kelvin, respectively
We need to convert the temperatures from Celsius to Kelvin by adding 273.15 to each value.
Given:
V1 = 550 mL
T1 = -55 °C = 218.15 K
T2 = 30 °C = 303.15 K
Assuming the pressure remains constant, we can rearrange the equation to solve for V2:
V2 = (P1 * V1 * T2) / (P2 * T1)
Since the pressure is not specified in the problem, we can assume it remains constant, allowing us to cancel out the pressure terms. Thus, the final equation becomes:
V2 = (V1 * T2) / T1
Plugging in the given values:
V2 = (550 mL * 303.15 K) / 218.15 K
Simplifying the calculation, we find:
V2 ≈ 760.67 mL
Therefore, the volume of the gas at 30 °C is approximately 760.67 mL.
For more question on gas law
https://brainly.com/question/27870704
#SPJ8
Related Questions
2.
If your favorite radio station has a frequency of 103.5 MHz, what is the wavelength
(in meters) of the radiation emitted by this station?
Answers
The wavelength of lambda is 2.93 m. = (c)/(v) = (3 xx 10(8) ms-(-1), 102.5 xx 10(6)s-(-1), 2.93 m' 2.93 meters is the wave length. 27 Jun 2022.
How can the wavelength of a radio wave be calculated in meters?In order to get a radio wave's wavelength, divide its frequency by the speed of light (c = 299 792 458 m). The following is the formula for determining a radio wave's wavelength: Wavelength is equal to c/f, where c is the speed in meters per second, and f is the frequency in Hertz.
How is a wavelength calculated?A wave train's speed (v) in a medium is equal to its frequency (f), hence the formula for wavelength is v/f. Wavelength is typically represented by the Greek symbol lambda ().
To know more about wavelength visit :-
https://brainly.com/question/13533093
#SPJ1
What is the correct order for the first three steps of the scientific method?
A. State the question, conduct an experiment, form a hypothesis
B. Form a hypothesis, form a conclusion, conduct an experiment
C. Conduct an experiment, form a hypothesis, analyze the data
D. State the question, form a hypothesis, conduct an experiment
SUBMIT
Answers
Answer:
D.) State the question, form a hypothesis, conduct an experiment
Explanation:
https://www.colorincolorado.org/article/steps-scientific-process
5. (a-c) In the balanced equation below, how many grams of solid iron can be formed if 50.0g Fe2O3 reacts with 10.0g of CO?
Fe2O3(s) + 3CO(g) → 2Fe(s) +3CO2
a. What mass of the excess reactant remains after the reaction occurred?
b. If you conducted an experiment and 11.5 grams of Fe(s) was formed what would be the percent yield?
c. If the percent yield for the process to obtain 75 kg of CO2 was 80.7%, how many kg of CO is needed?
Answers
From the balanced equation of the reaction, the mole ratio of the reactants is 1:3.
Mole of 50.0 g \(Fe_2O_3\) = 50/160 = 0.3125 mol
Mole of 10.0 g CO = 10/28 = 0.3571 mol
Stoichiometric equivalent of \(Fe_2O_3\) = 0.3571/3 = 0.1190 mol
In other words, \(Fe_2O_3\) is in excess.
Excess mole = 0.3125 - 0.1190 = 0.1935 mol
Mass of 0.1935 mol \(Fe_2O_3\) = 0.1935 x 160 = 30.96 grams
Mole ratio of CO to Fe = 3:2
Equivalent mole of Fe that would be formed = 0.3571 x 2/3
= 0.2381 mol
Mass of 0.238 mol Fe = 0.238 x 56 = 13.328 grams
Percent yield of Fe = 11.5/13.328 x 100 = 86.28%
Mole of 75 gk CO2 = 75000/44 = 1704.55 mol
Mole ratio of CO2 and Co = 1:1
Equivalent mole of CO = 1704.55 mol
Mass of 1704.55 mol CO = 1704.55 x 28 = 47.73 kg
80.7% = 47.73 kg
100% = 100 x 47.73/80.7
= 59.14 kg
In other words, 59.14 kg of CO would be needed.
More on stoichiometric problems can be found here: https://brainly.com/question/29856106
#SPJ1
Look at the picture and observations below.
Observations: The bee's wings are moving very fast.
The bee's wings are much smaller than its body.
what’s the answer ?
Answers
Answer:
How are bees able to fly?
Explanation:
An aqueous solution (water is the solvent) of a KCl compound ( l = 2 ) boils at 102.5 degrees celcius. What is the molality (m) of the solution? Assume that the pure water boils at 100 degrees celcius.
Answers
Explanation
Given:
i = 2
boiling point of water = 100 'C
KCl boiling point = 102.5 'C
Required: Molality (m) of the solution
Solution
DT = i x Kb x m
DT = 102.5-100 = 2.5 'C
DT = i x Kb x m
2.5 = 2 x 0.512 x m
2.5 = 1.024m
molality = 2.44 m
Answer
Molality = 2.44 m
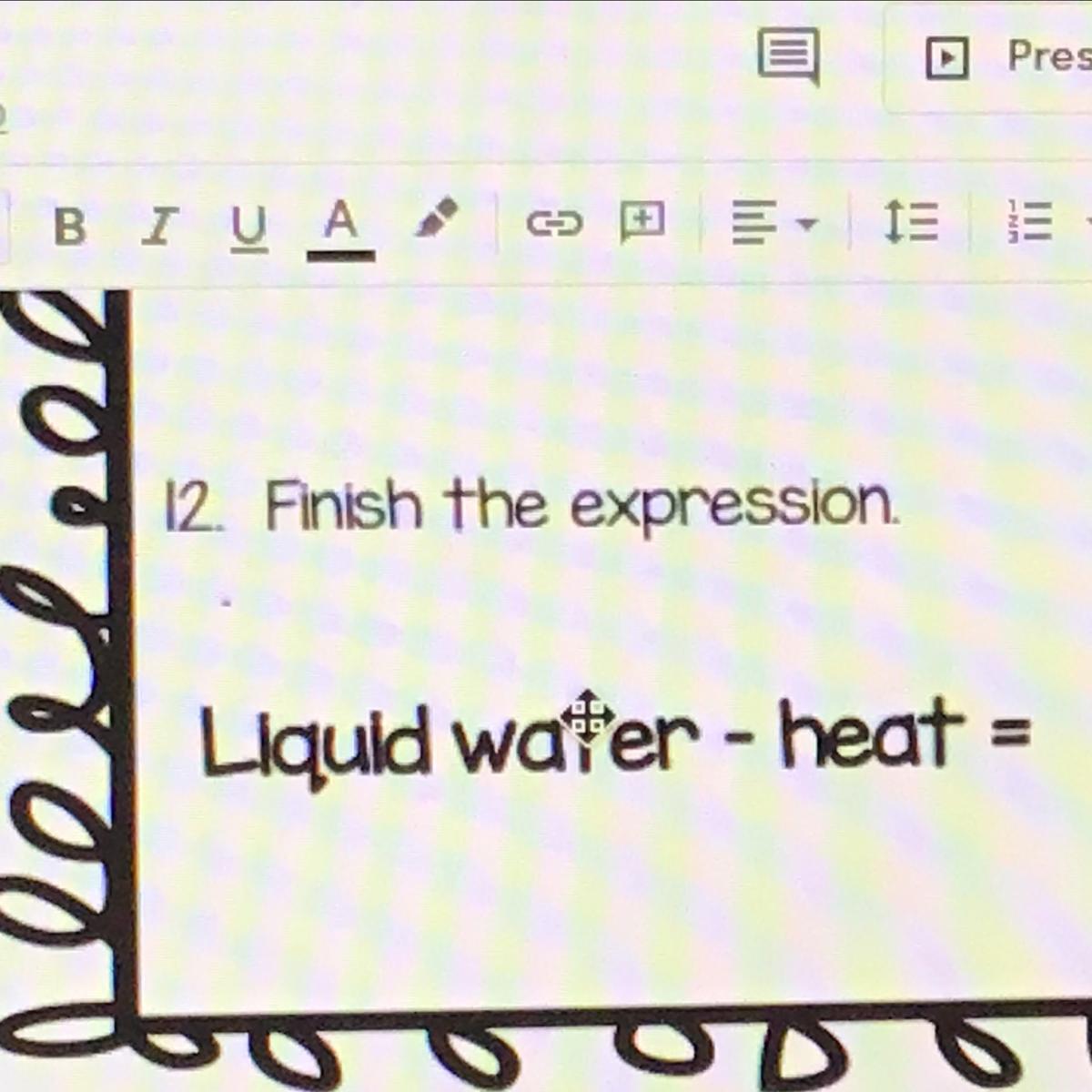
Liquid water - heat =
Pls help now!!!
Answers
Answer:
cold or ice?
Explanation:
have a good day.
The lethal dose of aspirin is 50 mg per kg of body weight. How many 325 mg tablets would be deadly for a 60 lb child?
Answers
To determine the number of 325 mg tablets of aspirin that would be deadly for a 60 lb child, we first need to convert the weight to kg.
1 lb is equal to 0.453592 kg. Therefore, a 60 lb child weighs approximately 27.2155 kg (60 x 0.453592).
The lethal dose of aspirin is 50 mg per kg of body weight. Therefore, for a 27.2155 kg child, the lethal dose would be 1,360.775 mg (27.2155 x 50).
Each aspirin tablet is 325 mg. Therefore, the number of tablets that would be deadly for the child would be 4.19 (1,360.775 / 325).
However, it is important to note that even a slightly higher dose of aspirin can be harmful to a child, and it is never recommended to give aspirin to children without consulting a doctor first.
To know more about aspirin, visit :
https://brainly.com/question/29133232
#SPJ1
Which subatomic particle is used to identify the atom, as it never changes?
Protons
Electrons
Neutrons
Nucleus
Answers
Explain two positive aspects of using methane recapture systems.
Answers
Answer:
Two positive aspects of using methane recapture systems are able to generate significant electricity. Another benefit is that the process of anaerobic digestion creates heat that can be used to warm buildings where animals are kept
Answer: The correct answer is;
Two positive aspects of using methane recapture systems include lowering the impact on greenhouse gasses and the production of energy. Methane is a very potent greenhouse gas that is contributing to global warming. As a result, the recapturing process reduces the methane impacts of global warming by reclaiming and reusing the gas for other purposes. Recaptured methane can be stored and used to generate electricity or used as fuel to power updated vehicles and other engines on the farm. The overall benefits from this combination are reducing impacts causing global warming and lower the cost of electricity or fuel on the farm.
Explanation: This answer has been confirmed correct.
A. 3
B. 4
C. 7
D. 10
WILL GIVE BRAINSLIST

Answers
Answer:
C. 7
Explanation:
act what's the question?
what is the change in mass of A in
60 minutes?
Mass of A (g)
12.4
10.4
9.1
7.7
6.2
Time
O
15
30
45
60
Answers
Answer:
To determine the change in mass of A over the given time period, we need to find the difference between the initial mass of A and the final mass of A.
From the given table, we can see that the initial mass of A at t = 0 (start time) is 12.4 g and the final mass of A at t = 60 minutes (end time) is 6.2 g.
Therefore, the change in mass of A over 60 minutes is:
Final mass of A - Initial mass of A
= 6.2 g - 12.4 g
= -6.2 g
The negative sign indicates that the mass of A decreased over time, which means that A underwent some kind of reaction or process that caused it to lose mass.
The change in mass of A over 60 minutes is -6.2 grams.
To determine the change in mass of A over 60 minutes, we need to compare the initial mass to the final mass.
From the given information, we can see that the mass of A decreases over time.
Let's calculate the change in mass.
Initial Mass of A: 12.4 g
Final Mass of A: 6.2 g
Change in Mass of A = Final Mass of A - Initial Mass of A
= 6.2 g - 12.4 g
= -6.2 g
The change in mass of A over 60 minutes is -6.2 grams.
Note that the negative sign indicates a decrease in mass.
For such more questions on mass
https://brainly.com/question/1838164
#SPJ8
Describe how lead as a toxic metal can be determine in borehole water?
Answers
We can be able to determine the amount of toxic lead in the water by thee use of atomic absorption spectrophotometry.
What is a toxic metal?
A toxic metal is known as any metal that is able to affect the health of people. We know that toxic metals are mostly the metals that are in the group of the heavy metals.
Now we know lead as a metal that is able to cause brain damage especially in children. This is why it is very important that there should be a thorough examination in order to know the amount of lead that is present in water.
There are several methods that could be applied in the determination of lead and one of the most common methods is by the use of atomic absorption spectrophotometry which is able to detect even the minutest amount of the led in solution.
Learn more about toxic metals:https://brainly.com/question/28331004
#SPJ1
2. Determine the atomic mass of sulfur given the following naturally occurring isotopes.
sulfur -32 is 94.99% and 31.972 amu,
sulfur -33 is 0.75% and 32.971 amu,
I
sulfur - 34 is 4.25% and 33.968 amu and
sulfur - 36 is 0.01% and 35.967 amu.
Answers
Answer:
32.06 amu
Explanation:
(0.9499 * 31.972) + (0.0075 * 32.971) + (0.0425 * 33.968) + (0.0001 *35.967) =
32.06
Considering the definition of atomic mass, isotopes and atomic mass of an element, the atomic mass of sulfur is 32.06 amu.
The isotopes of an element are those in which its atomic numbers (that is, the number of protons) are the same, but the number of neutrons is different. Remember that protons and neutrons are in the nucleus of the element.
When the mass of a chemical element is fractional, it indicates that said element will be made up of a mixture of its different isotopes. So the average atomic mass of an element is calculated based on the abundance and mass of its isotopes.
In this case, you know the following isotopes and its abundance:
94.99% (0.9499) - 31.972 amu 0.75% (0.0075) - 32.971 amu 4.25% (0.0425) - 33.968 amu 0.01% (0.0001) - 35.967 amuThen, the average atomic mass is calculated as:
atomic mass= 0.9499×31.972 amu+ 0.0075×32.971 amu + 0.0425×33.968 amu + 0.0001×35.967 amu
atomic mass= 32.06 amu
Finally, the atomic mass of sulfur is 32.06 amu.
Learn more:
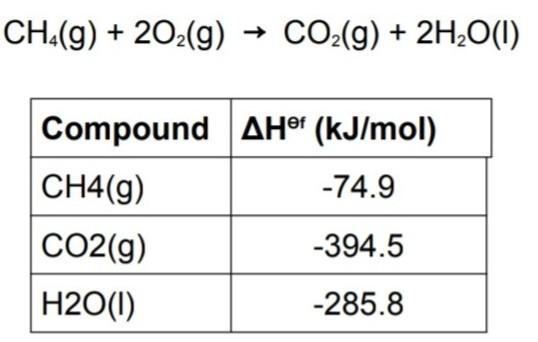
brainly.com/question/14403750?referrer=searchResults brainly.com/question/10043246?referrer=searchResults brainly.com/question/15553207?referrer=searchResultshttps://brainly.com/question/13596853Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
a ___ weather front forms when a warm air mass pushes into a cooler air mass
a) cold
b) warm
c) stationary
Answers
Answer:
b) warm
Explanation:
A warm weather front forms when a warm air mass pushes into a cooler air mass. Therefore, the answer is b) warm.
I'm sorry to bother you but can you please mark me BRAINLEIST if this ans is helpfull
1. What is the Kinetic Energy of a 150 kg object that is moving with a speed of 15 m/s?
Answers
Answer:
16875 J
Explanation:
KINETIC ENERGY EQUATION = 1/2 m v^2
= 1/2 times mass times velocity of metres per second^2 (speed)
= 1/2 times 150 times by 15^2
= 16875 J (joules)
Then, using information from the “Atomic Zoom-In” article, explain why two substances have different properties to a member of your household.
You may work with more than one member of your household.
You might need to explain a little about what properties are and the different properties the two substances have in order for your household member to be able to work with you.
When you are finished, ask the person what she learned about properties. Record the answer below.
What did your household member learn about properties?
Answers
Answer: Two substances have different properties because they are made of different types and numbers of atoms that repeat.
Explanation: According to the article “Atomic Zoom-In”, all matter is made of tiny pieces called atoms, and there are 118 different types of atoms in the universe. Every substance is made of a unique combination of atoms, which can be represented by a chemical formula. The chemical formula shows the types and numbers of atoms that repeat to make up a substance.
For example, water has a chemical formula of H2O, which means it is made of groups of 2 hydrogen atoms and 1 oxygen atom. Substances have different properties because they are made of different types and numbers of atoms that repeat.
For example, water and ethanol are both clear liquids, but they have different properties such as boiling point, density, and flammability. This is because water is made of H2O groups, while ethanol is made of C2H6O groups.
The different types and numbers of atoms affect how the molecules interact with each other and with other substances, resulting in different properties. Therefore, to explain why two substances have different properties, we need to look at their chemical formulas and see how their atoms differ.
Hope this helps, and have a great day! =)
if the reaction used up 2.35 moles of H2 , how many moles of NH3 were produced? use this eqationN2 + 3 H2 → 2 NH3
Answers
Answer:
mark me brilliant
Explanation:
According to the balanced chemical equation, for every 3 moles of H2 consumed, 2 moles of NH3 are produced.
Therefore, to find the number of moles of NH3 produced, we need to determine the ratio of H2 to NH3 based on the balanced equation:
3 moles H2 : 2 moles NH3
If 3 moles of H2 produces 2 moles of NH3, then 2.35 moles of H2 would produce:
(2 moles NH3 / 3 moles H2) x 2.35 moles H2 = 1.57 moles NH3
So, 1.57 moles of NH3 would be produced if 2.35 moles of H2 were consumed in this reaction.
A gas has a volume of 6.00 liters at a temperature of 27° C and a pressure of 1,00 atm. What is the volume of the gas, in liters, at a temperature of 327°C and a
pressure of 3.00 atm?
Answers
We are given:
P1 = 1 atm P2 = 3 atm
T1 = 300 K T2 = 600 K
V1 = 6 L V2 = v L
Finding the final Volume:
From the ideal gas equation:
PV = nRT
since in the given scenario, the universal gas constant (R) and number of moles(n) are constant
So, for both the cases, the value of n*R will be a constant k
Hence, we can write that:
PV / T = k [where k is a constant]
Since the constant 'k' is the same for both the cases, we can write that:
P1V1 / T1 = P2V2 / T2
replacing the variables
1 * 6 / 300 = 3 * v / 600
1/50 = v / 200
v = 200/50
v = 4 L
Therefore, the volume of the gas at 600K and 3 atm will have a volume of 4 L
In the figure, which two lines appear to be parallel?
Answers
Which statement is true regarding weathering in drying environments?
Answers
Answer:
weathering in dry climates can be erosion of sand hitting against rocks or whatever there may be.
Explanation:
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Calculate the number of moles
309 grams of (SF)4 = how many moles of (SF)4
Answers
Answer:
2.85 mol
Explanation:
Step 1: Given data
Mass of sulfur tetrafluoride (SF₄): 309 g
Step 2: Calculate the number of moles corresponding to 309 g of sulfur tetrafluoride
To convert mass to moles we need a conversion factor: the molar mass. The molar mass of SF₄ is 108.07 g/mol.
309 g × 1 mol/108.07 g = 2.85 mol
1 x 3 2 9. Find the value of x if-1 -1 2 | 04-21 10. Sobre the linear system using Cramer's rule = 16
Answers
The value of x is 1.
To solve the linear system using Cramer's rule, we need to find the value of x in the equation 1x + 3(2) + 9 = 16.
Simplifying the equation, we have:
x + 6 + 9 = 16
x + 15 = 16
x = 16 - 15
x = 1
Therefore, the value of x is 1.
Cramer's rule is a method used to solve systems of linear equations by using determinants. In this case, we have a single equation with one variable, so the determinant involved is a 1x1 matrix. The determinant of a 1x1 matrix is simply the value of the element.
The determinant of the coefficient matrix in this equation is 1. The determinant of the entire system is the determinant of the coefficient matrix divided by the determinant of the matrix formed by replacing the column of the variable with the constants.
Since the determinant of a 1x1 matrix is the value of the element, we have:
determinant of the entire system = determinant of the coefficient matrix / determinant of the constant matrix
= 1 / 1
= 1
Therefore, the determinant of the entire system is 1.
Using Cramer's rule, we can solve for the value of x by dividing the determinant of the matrix formed by replacing the column of the variable with the constants by the determinant of the coefficient matrix. In this case, both determinants are 1. Thus, The value of x is 1.
For more such questions on value
https://brainly.com/question/30371221
#SPJ8
The period of a wave is directly proportional to the wavelength of the wave. True or False
Answers
The period of a wave is directly proportional to the wavelength of the wave. True
What is the relationship between a wave's wavelength and its period?Period is the time it takes to complete one cycle of a wave, and wavelength is the distance between two identical locations in the neighbouring cycles of a wave. The number of cycles in a second is defined as frequency. In other terms, frequency = 1 / period.
An electromagnetic wave's wavelength is proportional to its frequency. Likewise, an electromagnetic wave's frequency is equal to the reciprocal of its period.
learn more about period of a wave
https://brainly.com/question/22059232
#SPJ1
1. Write a balanced equation for each of the following reactions. Be sure to include the state of matter for each reactant and product.
b) Solid calcium cyanide and liquid water react to generate calcium hydroxide and hydrogen cyanide, both in solution.
Answers
The balanced equation for the reaction between solid calcium cyanide and liquid water to generate calcium hydroxide and hydrogen cyanide would be \(Ca(CN)_2 (s) + 2H_2O(l)-- > Ca(OH)_2 (aq) + 2HCN (aq)\)
Balancing chemical equationsThe reaction between solid calcium cyanide and liquid water to generate calcium hydroxide and hydrogen cyanide would be written as follows:
The chemical formula of solid calcium cyanide = \(Ca (CN)_2 (s)\)
The chemical formula of liquid water = \(H_2O (l)\)
The chemical formula of calcium hydroxide in solution = \(Ca(OH)_2 (aq)\)
The chemical formula of hydrogen cyanide in solution = \(HCN (aq)\)
Bringing all the species together, the equation for the reaction would be:
\(Ca(CN)_2 (s) + H_2O(l)-- > Ca(OH)_2 (aq) + HCN (aq)\)But the above equation is not balanced. The number of hydrogen and cyanide atoms is not balanced. Balanced chemical equations always have an equal number of every atom in the reactants and in the products.
Thus, the balanced equation of the reaction would be;
\(Ca(CN)_2 (s) + 2H_2O(l)-- > Ca(OH)_2 (aq) + 2HCN (aq)\)
More on balancing chemical equations can be found here: https://brainly.com/question/28294176
#SPJ1
How many atoms are in 20.5 mg (miligram) argon.
Express answer in scientific notation
Answers
Answer:
3.09 x 10²⁰ atoms
Explanation:
We want to find the number of atoms in 20.5mg of argon.
We can use dimensional analysis to do so.
See attached image for table.
Needed conversions:
1g = 1000mg1 mol of Ag = 39.948g 1 mol = 6.022 x 10¹³ atoms
Perform the following conversions: (Show work on back of page for this problem only). 98.6 oF = __________ oC = __________ K _________ oF = 25.0 oC = __________ K _________ oF = __________ oC = 405 K
Answers
The conversion of the given units are as follows:
98.6oF = 37°C = 310 K = 98.33°F
25.0°C = 298K = 76.73°F
131.85°C = 405 K
What is temperature ?
Temperature is a physical quantity that quantifies our feelings of hotness and coldness. A thermometer is used to measure temperature.
According to the International System of Units, the SI unit of temperature is Kelvin, which is represented by the symbol K. In the fields of science and engineering, the Kelvin scale is widely accepted or used.
However, in most parts of the world, temperature is measured using the Celsius or Fahrenheit scale.
Thus, The conversion of the given units are 98.6oF = 37°C = 310 K = 98.33°F.
To learn more about the temperature, follow the link;
https://brainly.com/question/11464844
#SPJ1
If 40.5 J of heat is added to a 15.4 g sample of silver, what will the change in temperature be? (Specific heat of silver is 0.235 J/g °C
Please hurry it’s due in 10 min :/
Answers
Answer:
623.7
Explanation:
40.5*15.4=623.7
Solid ammonium chloride, NH4Cl, is formed by the reaction of gaseous ammonia, NH3, and hydrogen chloride, HCl. NH3(g)+HCl(g)⟶NH4Cl(s) A 6.63 g sample of NH3 gas and a 6.63 g sample of HCl gas are mixed in a 1.00 L flask at 25 ∘C. What is the pressure in atmospheres of the gas remaining in the flask? Ignore the volume of solid NH4Cl produced by the reaction.
Answers
9.41 atm is the pressure in atmospheres of the gas remaining in the flask
What is the pressure in atmospheres?The equation NH3(g) + HCl(g) ==> NH4Cl(s) is balanced.
Divide the moles of each reactant by its coefficient in the balanced equation, and the limiting reagent is identified as the one whose value is less. With the issue we now have...
6.44 g NH3 times 1 mol NH3/17 g equals 0.3688 moles of NH3 ( 1 = 0.3688)
HCl: 6.44 g of HCl times one mole of HCl every 36.5 g equals 0.1764 moles ( 1 = 0.1764). CONTROLLING REAGENT
NH4Cl will this reaction produce in grams
0.1764 moles of HCl multiplied by one mole of NH4Cl per mole of HCl results in 9.44 g of NH4Cl (3 sig. figs.)
the gas pressure, measured in atmospheres, that is still in the flask
NH3(g) plus HCl(g) results in NH4Cl (s)
0.3688......0.1764............0..........
Initial
-0.1764....-0.1764........+0.1764...Change
Equilibrium: 0.1924.......0...............+0.1924
There are 0.1924 moles of NH3 and no other gases in the flask. This is at a temperature of 25 °C (+273 = 298 °K) in a volume of 0.5 L. After that, we may determine the pressure by using the ideal gas law (P).
PV = nRT
P = nRT/V = 0.1924 mol, 0.0821 latm/mol, and 298 Kmol / 0.5 L
P = 9.41 atm
9.41 atm is the pressure in atmospheres of the gas remaining in the flask
To learn more about balanced equation refer to:
https://brainly.com/question/11904811
#SPJ1