A gamma ray has a frequency of 1 x 10^20 Hz and has a speed of c. What is the
wavelength?

Answers
Answer:
λ = 3.0 × 10⁻¹² m
Explanation:
Speed of Light = Wavelength × frequency
c = λν
Step 1: Define
c = 3.0 × 10⁸ m/s
λ = unknown
ν = 1 × 10²⁰ Hz
Step 2: Substitute and Evaluate for λ
3.0 × 10⁸ m/s = λ · 1 × 10²⁰ Hz
λ = 3.0 × 10⁸ m/s ÷ 1 × 10²⁰ Hz
λ = 3.0 × 10⁻¹² m
Related Questions
PLEASE help — I’m failing!
Determine each of the following for a 4.23 x 10-3 M solution of HCl:
a. H3o+
b. OH-
c. pH
d. pOH
Answers
Answer:
a.
\([H _{3}O {}^{ + } ] = 4.23 \times {10}^{ - 3} M\)
b.
\([OH {}^{ - } ] = \frac{kw}{[H {}^{ + } ]} \\ [OH {}^{ - } ] = \frac{1 \times {10}^{ - 14} }{4.23 \times {10}^{ - 3} } \\ [OH {}^{ - } ] = 2.36 \times {10}^{ - 12} M\)
c.
\(pH = - log[H {}^{ + } ] \\ pH = - log(4.23 \times {10}^{ - 3} ) \\ pH = 2.37\)
d.
\(pOH = - log[OH {}^{ - } ] \\ = - log(2.36 \times {10}^{ - 12} ) \\ = 11.63\)
Help me about it please

Answers
Answer:
4 atoms of Hydrogen
1 atom of Sulphur
4 atoms of Oxygen
hope it helps you
make me brainliest plz
Each of the following sets of quantum numbers is supposed to specify an orbital. Choose the one set of quantum numbers that does not contain an error. Select one: a. n = 3,1 = 2, ml = +3 b. n = 4, 1 = 0, ml = -1 c. n = 3, 1 = 1, ml = -2 d. n = 4,1 = 4, ml = 0 e. n = 5,1 = 3, ml = -3
Answers
The quantum numbers does not contain an error is n = 5, l = 3, ml =-3 .
What is meant by quantum numbers ?Quantum numbers in quantum physics and chemistry explain the values of conserved quantities in a quantum system's dynamics. Quantum numbers are quantities that may be precisely known together with the energy of the system and their corresponding eigenspaces. They correspond to the eigenvalues of operators that transact with the Hamiltonian. A base state of a quantum system is fully described by the specification of all of its quantum numbers, which may theoretically be measured collectively.The quantization of several interesting observable quantities is a crucial component of quantum physics. This specifically results in quantum numbers, though they may occasionally approach infinity, that take values in discrete sets of integers or half-integers.Learn more about quantum numbers refer to :
https://brainly.com/question/2292596
#SPJ4

Draw the structures of the following amino acids as they would appear in solution of pH 10. A) lysine, B) glutamic acid, C) alanine, D) glycine
Answers
The question requires an understanding of the protonation and deprotonation states of the amino and carboxyl groups in these amino acids under basic conditions, as well as the chemical properties of their R-groups.
How to describe the structures of four different amino acids, namely lysine, glutamic acid, alanine, and glycine, as they would appear in a solution of pH?
A) Lysine: At pH 10, lysine would be in its protonated form, with a positive charge on the amino group (-NH3+). The carboxyl group (-COO-) would be deprotonated, giving it a negative charge. The R-group of lysine is a long hydrophobic chain with a terminal amino group (-CH2-CH2-CH2-CH2-CH2-CH2-CH2-NH2), which would also be protonated at pH 10.
B) Glutamic acid: At pH 10, glutamic acid would be in its deprotonated form, with a negative charge on the carboxyl group (-COO-). The amino group (-NH3+) would be protonated, giving it a positive charge. The R-group of glutamic acid is a carboxylic acid (-CH2-CH2-COOH), which would also be deprotonated at pH 10.
C) Alanine: At pH 10, alanine would be in its protonated form, with a positive charge on the amino group (-NH3+). The carboxyl group (-COO-) would be deprotonated, giving it a negative charge. The R-group of alanine is a simple methyl group (-CH3), which is non-polar and does not carry any charge.
D) Glycine: At pH 10, glycine would be in its protonated form, with a positive charge on the amino group (-NH3+). The carboxyl group (-COO-) would be deprotonated, giving it a negative charge. The R-group of glycine is a simple hydrogen atom, making it the smallest amino acid and non-polar.
Learn more about structures of alanine and glycine at pH 10
brainly.com/question/17218267
#SPJ11
What determines an element's properties?
ANSWER: A. The valence electrons
A. The valence electrons
B. The isotopes it forms
C. Its atomic mass
D. The core electrons
Answers
Answer:
b
Explanation:
why does the wind have a tendency to flow parallel to the isobars above the friction level?
Answers
The wind tends to flow parallel to the isobars above the friction level due to the balance between the pressure gradient force and the Coriolis force.
The pressure gradient force is responsible for the wind's movement from areas of high pressure to areas of low pressure. Isobars are lines connecting points of equal pressure on a weather map. The closer the isobars are to each other, the steeper the pressure gradient and the stronger the pressure gradient force.
The Coriolis force is an apparent force resulting from the Earth's rotation. It deflects the wind to the right in the Northern Hemisphere and to the left in the Southern Hemisphere. The Coriolis force increases with increasing wind speed.
Above the friction level, where the influence of surface roughness is minimal, the pressure gradient force and the Coriolis force are the primary factors determining wind direction. The wind tends to flow parallel to the isobars because the pressure gradient force is balanced by the Coriolis force. This balance results in a geostrophic wind flow, which is characterized by winds moving parallel to the isobars at a constant speed.
In conclusion, above the friction level, the wind flows parallel to the isobars due to the balance between the pressure gradient force and the Coriolis force. This geostrophic wind flow pattern is observed in the upper levels of the atmosphere where the influence of surface friction is minimal.
To know more about Coriolis force visit:
brainly.com/question/30379412
#SPJ11
what occurs when ice melts? responses a physical change a physical change an atomic compound is newly formed. an atomic compound is newly formed. a nuclear change a nuclear change a chemical change
Answers
When ice melts a physical change occur. The correct answer is A.
The capacity to flow gives an ice cube the ability to alter shape as it melts. Its makeup stays the same, though. One instance of a physical change is melting. A physical change is when a sample of matter experiences a change in some of its qualities but not in its identity.
A reversible change in the substance's or object's physical characteristics can be referred to as the physical change. It is a physical change when ice melts. Water (H₂O) appears to alter when it turns from a liquid state to a solid state (ice). However, this shift is just physical, as the constituent molecules still consist of 11.19% hydrogen and 88.81% oxygen by mass.
Learn more about physical change at https://brainly.com/question/28742279
#SPJ4
Which of these is NOT a way to express probability?
A .1 in 4
B. 50 percent
c. 3/4
D. 25
Answers
Answer:
Option A.
Explanation:
Probability is expressed in percent, fraction or in whole numbers
The only one that does not express a probability is 25.
A one in four chance, a fifty percent chance, a three in four chance. These all could be probabilities.
25 doesn't express a probability.
If this is incorrect, please, don't refrain to tell me.
you have two compounds, a and b that are enantiomers of one another. b rotates like 12.5 degrees. you measure the optical activity of a mixture of a and b and determine that the rotation of plane polarized like is -3.6 degrees. what percentage of the enantiomers in solution are compound a?
Answers
Percentage of the enantiomers in solution are compound A = 78.27%
B rotates +12.5 degrees then A rotates -12.5 degrees
Enantiomeric excess = Specific rotation of mixture / specific rotation of pure enantiomer * 100
EE = -3.6/-12.6*100 = 28.57 %
percentage of A = 50 + 28.57 = 78.27 %
Enantiomers are a pair of molecules that can never be superimposed one on top of the other despite existing in two mirror images of one another. Enantiomers are chemically similar to one other in all other respects. The phrase "optical isomers" refers to a pair of enantiomers that can be identified by the direction in which, when dissolved in solution, they rotate polarized light, either dextro (d or +) or levo (l or -). A racemic combination is one that does not spin polarized light when two enantiomers are present in equal amounts because the optical activity of each enantiomer is cancelled out by the other.
Learn more about Enantiomers here:
https://brainly.com/question/21881808
#SPJ4
3. Which of the following provides the best explanation for why the water drop does not slide off the inclined plane?
90 80 70
50
40
30
10
O A The polar water molecules are absorbed by the underlying surface.
OB The polar water molecules cause the surface to become temporarily charged, causing adhesion
OC The polar water molecules exert strong cohesive forces on one another
OD. The polar water molecules are repelled by the nonpolar surface
Answers
Answer:
The polar water molecules exert strong cohesive forces on one another
Explanation:
The forces of cohesion refer to the strong attractive forces that molecules of a substance exert on each other.
This strong attractive force keeps the molecules of the water together and causes the water molecules to be pulled inside towards each other. We refer to this phenomenon as surface tension.
Hence, due to surface tension, water does not run off an inclined plane.
average atomic mass473homean element has two stable isotopes. one has a mass of 6.015 amu and is 7.59% abundant. the second has a mass of 7.016 amu and is 92.41% abundant. what is the average atomic mass of the element? blank atomic mass units. average atomic mass
Answers
The average atomic mass of the given element is 6.877 atomic mass units (amu).
We have been given the following data about the isotopes of the element:
Mass of first isotope, m₁ = 6.015 amu
Abundance of first isotope, a₁ = 7.59% or 0.0759
Mass of second isotope, m₂ = 7.016 amu
Abundance of second isotope, a₂ = 92.41% or 0.9241
Using the formula for average atomic mass of an element, we have:
average atomic mass = (m₁ × a₁) + (m₂ × a₂)
average atomic mass = (6.015 amu × 0.0759) + (7.016 amu × 0.9241)
average atomic mass = 0.4562 amu + 6.4665 amu
average atomic mass = 6.9227 amu
Rounding off to the nearest hundredth, we get:
average atomic mass ≈ 6.88 amu
Therefore, the average atomic mass of the given element is 6.877 atomic mass units (amu).
learn more about atomic mass here
https://brainly.com/question/30390726
#SPJ11
TMS 40 20 PPM 180 60 140 60 140 12010080 160 FIG. 48.8 The IR spectrum of cis-norbornene- 5,6-endo-dicarboxylic anhydride. Microns (um) 5.0 6.0 8 10 15 20 2.5 3.0 3.5 4.0 1838 cm 1759 cm-1 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm-1)
Answers
There are two peaks for anhydride at the above freq. our peaks are at 1759 cm-1 and one at 1838 cm-1 between two carbony1 group increases the freq. while resonance lowers the frequency.
peak at 1100cm-1 C-O
PEAK at 1600 CM-1 alkene
2850 CM-1-3000 CM-1 ALKANE STRETCH.
3150 CM-1 ALKANE STRETCH
While cracking the dimer it will give us monomer which then reacts with maleic anhydride to give Diels-Alder product. It does not give any unwanted product at higher temperature. If monomer reaction was low temperature and cracked.
Breaking the dimer at high temperature and it is to be reacted immediately can isolate the pure product.
To learn more about IR spectrum
https://brainly.com/question/29754365
#SPJ4

Why does increasing temperature generally increase the rate of a chemical reaction?
A. It increases the amount of reactant
B. It decreases the size of the reactant particles.
C. It causes the particles of the reactants to move faster and collide more often.
D. It increases the space between reactant particles so they have more room to move apart
Answers
What is the process that involves the breaking of intermolecular forces that hold an enzyme into the ordered three dimensional shape?
Answers
The process that involves the breaking of intermolecular forces that hold an enzyme is known as denaturation.
What are enzymes?
A substance produced by a living organism which acts as a catalyst to bring about a specific biochemical reaction.Almost all enzymes are proteins. There are some nucleic acids that behave like enzymes. These are called ribozymes. An enzyme like any protein has a primary structure, i.e., amino acid sequence of the protein. An enzyme like any protein has the secondary and the tertiary structure. A tertiary structure that contains the backbone of the protein chain folds upon itself, the chain criss-crosses itself and hence, many crevices or pockets are made. One such pocket is the ‘active site’. An active site of an enzyme is a crevice or pocket into which the substrate fits. Thus enzymes, through their active site, catalyse reactions at a high rate.To learn more about enzymes: https://brainly.com/question/1996362
#SPJ4
What name should be used for the ionic compound Cu
Answers
i’m not sure if this is 100% right!!
Its molecular formula is Cu+2
How much energy is released when a peanut is burned in a calorimeter? The temperature of 1.00Kg of water rose from 22.6°C to
26.5°C. The specific heat of water is 4.184J/g °C.
Answers
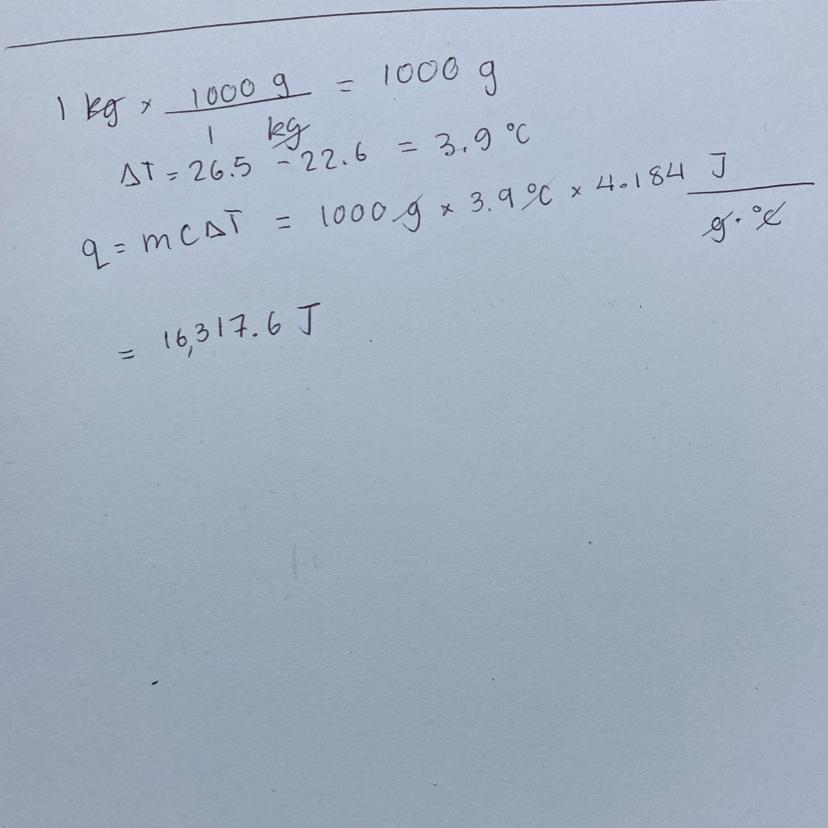
You work in the special effects department of a movie studio. You are
currently working on a superhero movie where the hero is very strong
and can punch through metal. For the next scene you need to replace a
6 inch by 6 inch square of a metal wall with a different material that will
crumble when the actor hits it. What could you use?
A. You could use Carbon(C)
B. You could use Potassium (k)
C. You could use Titanium (T)
D. You could use Manganese (Mn)
Answers
Answer:
The correct option is;
D. Manganese (Mn)
Explanation:
Manganese is very brittle, hard, iron like silvery-gray metal, that is difficult to melt. In air, Manganese slowly disintegrate in a similar manner to iron rusting in water
Manganese and iron have similar chemical and physical properties however manganese is more harder and more brittle than iron
A brittle material is one that easily breaks without deforming elastically
Therefore, manganese, due to its very iron like appearance and brittle nature will be suitable to replace the metal wall and crumble easily when the actor hits it.
directions: this group of questions consists of five lettered headings followed by a list of phrases or sentences. for each phrase or sentence, select the one heading to which it is most closely related. each heading may be used once, more than once, or not at all. (a) glycolysis (and) krebs cycle (citric acid cycle) (c) calvin cycle (light-independent reactions of photosynthesis) (d) light-dependent reactions of photosynthesis (e) chemiosmosis question process in which co2, is released as a by-product of oxidation-reduction reactions
Answers
Glycolysis is the most closely linked process, according to the remark made.
What is the main function of glycolysis?Proteolysis is the first method by which molecules are broken down to produce energy. This activity takes place in the cytosol from both prokaryotes. This became likely one of the earliest digestive routes to evolve because nearly all life on Earth employs it.
What are the stages of glycolysis?The initial process in breaking down glucose to release energy for the cell's activity is called glycolysis. Glycolysis consists of a fuel step and an electricity phase. Sugar is converted to provide energy at an expense of one ATP. Step 2: An hydrolase converts glucose 5-phosphate into alcohol hydrolysed, which is its isomer.
To know more about glycolysis visit:
https://brainly.com/question/14076989
#SPJ4
How did fossil fuels get there name
Answers
Answer:
Explanation:
Fossil fuels got their name because they are made from dead organisms, mostly plants that didn't decay because they were squashed under water or mud with no oxygen.
3)What helps the plants to receive sunlight in tropical rainforests?
Answers
Answer:
Large leaves help plants to receive more sunlight when in tropical rainforests.
is hardness physical or chemical property
Answers
Answer:
The properties of the Hardness are:
Hardness is a physical property
Hardness is indicative of the strength of chemical bonds between elements.
Diamond can scratch quartz Explanation:
Is the Earth's surface covered with the same materials?
What was different about each scientific drilling site?
What is the same for all of the drilling sites we examined?
Answers
The Earth's surface is not covered with the same materials ; however, some areas share similar materials.Several drilling sites have been dug in various regions of the planet to analyze the Earth's surface.
Each drilling site is unique, with differing characteristics and results. Despite these differences, all of the drilling sites analyzed offer scientists a more in-depth knowledge of the Earth's surface.In essence, the scientific drilling sites each had different lithologies, stratigraphies, and geologies. Each site had different types of rocks, depths, and ages, which led to varying drilling conditions, depths, and equipment used. Different types of equipment were also used to reach the depths required, which was a significant difference in each drilling site.The scientific drilling sites also had different purposes. Scientists had specific goals they wanted to achieve at each location. For example, the scientific drilling site in the Iceland region was focused on analyzing a unique layer of igneous rocks. The primary objective was to investigate the formation of the rock layer.The same materials were not found at each scientific drilling site.
Still, they had some similarities. They all provided geologists with vital information about the Earth's surface. The data provided from each drilling site was used to piece together the Earth's geologic history and how it has changed over time.
for such more questions on surface
https://brainly.com/question/30116754
#SPJ8
How many moles of CO² are produced from the combustion of 6.4 mol C³H8?
Answers
The balanced chemical equation for the combustion of C3H8 is:
C3H8 + 5O2 → 3CO2 + 4H2O
According to the equation, for every 1 mole of C3H8 that is burned, 3 moles of CO2 are produced.
Therefore, to find the number of moles of CO2 produced from the combustion of 6.4 mol of C3H8, we can use the mole ratio from the balanced equation:
6.4 mol C3H8 × (3 mol CO2 / 1 mol C3H8) = 19.2 mol CO2
So, 19.2 moles of CO2 are produced from the combustion of 6.4 mol of C3H8.
Limestral flour (calcium carbonate) is a weak base used for at a few years of intervals Neutralize the acidification that takes place by lakes and woodland. Would not be a good idea that instend Using the stronger base sodium hydroxide and then be able to use a little less amount?
Answers
Answer:
Well, no.
Explanation:
NaOH is a base which is found in soap, and other detergents which gives it the slippery feel, because, it is a really strong base. Applying this to lakes and woodland could make the water and woodland really concentrated which could make them harmful for domestic and industrial use
75.000 ml of helium at stp, using significant figures how many moles of helium are contained within the ballon
Answers
Using significant figures 0.00296 moles of helium can be calculated to be contained in the balloon.
How do you calculate the moles of helium that are contained within the balloon?You might have learnt in school that at STP (Standard Temperature and Pressure), the temperature is 273.15 K and the pressure is 1 atm. The molar volume of any given gas at STP will always be 22.4 L/mol. This will perhaps be known to you as Avogadro's Law.
You are having 75.000 mL of helium, which is 0.075 L. To find the number of moles of helium, you will make use of the ideal gas law:
PV = nRT
The notations are commonly known as P for pressure, V for volume, n is for number of moles, R for gas constant, and T for temperature in Kelvin scale.
At STP, P = 1 atm and T = 273.15 K. The gas constant R is 0.08206 L·atm/mol·K.
So you will then have:
n = PV/RT = (1 atm)(0.075000 L)/(0.08206 L·atm/mol·K)(273.15 K)
n = 0.00296 mol
Using significant figures, you can then round this to three significant figures:
n = 0.00296 mol
The numbre of moles is 0.00296. This is your final answer.
Learn more about Avogadro's Law here:
https://brainly.com/question/31216850
#SPJ1
Need help. Is this correct

Answers
Answer:
Yes, that is correct!
Explanation:
Answer:
Yes
Explanation:
A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electrons are simultaneously attracted by the two atomic nuclei. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions.
in the group the reactivity of metals increases? give example
Answers
Answer:
As sodium and potassium both are alkali metals that belong to Group IA of the periodic table. They have one valence electron in their valence shell. As we move from top to bottom, the reactivity of metallic elements of Group IA increases.
The novice nurse administers RBCs to a client. Which actions by the novice nurse are deemed safe by the nurse preceptor? (Select all that apply.)
Priming the intravenous tubing with 0.9% sodium chloride.
Obtaining and documenting a full set of baseline vital signs.
NOT setting the infusion rate to deliver blood within 6 hours - it should be 4 hours.
Also require large gauge catheters 20-24 gauge.
Should stay with client for first 15 minutes
Answers
According to the nurse preceptor, the new nurse adheres to a number of safe practices while administering red blood cells (RBCs) to a patient.
Based on the given options, the actions that are deemed safe by the nurse preceptor are:
Priming the intravenous tubing with 0.9% sodium chloride.Obtaining and documenting a full set of baseline vital signs.Setting the infusion rate to deliver blood within 4 hours instead of 6 hours.Using large gauge catheters (20-24 gauge). When giving red blood cells (RBCs) to a patient, the novice nurse follows a number of safe procedures, according to the nurse preceptor. To ensure appropriate flushing and lower the chance of an air embolism, the inexperienced nurse correctly primes the intravenous tube with 0.9% sodium chloride in the first step. The second step is for the inexperienced nurse to collect and record a complete set of baseline vital signs. This creates a baseline for monitoring the client's status both before and after the transfusion. Third, in accordance with the advised duration for safe administration, the nurse modifies the infusion rate to administer the RBCs in 4 hours as opposed to 6 hours. Fourth, the inexperienced nurse employs big gauge catheters (20-24 gauge) to promote quick and smooth blood product flow and reduce problems.
To learn more about RBC's, refer to:
https://brainly.com/question/19029068
#SPJ4
How many molecules are there in 3.20 grams of NH4SO2?? explain if you can
Answers
Answer:
0.0389757845886336
Explanation:
sales for adidas grew at a rate of 0.5196 in year 1, 0.0213 in year 2, 0.0485 in year 3, and −0.0387 in year 4. the average growth rate for adidas during these four years is the closest to __________.
Answers
The answer is 0.14.
To calculate the average growth rate for Adidas during the four-year period, we need to find the arithmetic mean of the individual growth rates. Here are the steps:
1. Sum up the growth rates for each year:
Sum = 0.5196 + 0.0213 + 0.0485 + (-0.0387)
2. Divide the sum by the total number of years (4 in this case):
Average Growth Rate = Sum / 4
By evaluating this expression, you can find the average growth rate for Adidas during the four-year period.
Total growth rate = 0.5196 + 0.0213 + 0.0485 - 0.0387 = 0.5507
Average growth rate = Total growth rate / Number of years = 0.5507 / 4 = 0.1377
Therefore, the closest answer is 0.14.
To know more about average growth rate, refer here
https://brainly.com/question/31724520#
#SPJ11