Answers
Answer:
Distance lift the tree = 0.5 m
Explanation:
Given:
Work done = 50 joules
Force = 100 newton
Find:
Distance lift the tree
Computation:
Distance = Work done / Force
Distance = 50 / 100
Distance = 0.5 m
Distance lift the tree = 0.5 m
Related Questions
Describe how naturally
acidic rainwater can affect a
mountain of limestone.
Answers
Answer:
The naturally acidic rain will gradually wear the mountain of limestone away.
Hope it helps!
Here is a second order reaction A→ P. If the initial concentration of A 0.0818 M goes down 30.0% in 3.15 minutes, what is the rate constant for the reaction?
Answers
The rate constant of the second-order reaction is 0.111 M^-1 min^-1.
The given data represents a second-order reaction where the rate of the reaction is proportional to the square of the concentration of A.
The integrated form of the second-order reaction is:
1/[A]t = kt + 1/[A]0
where [A]t and [A]0 are the concentrations of reactant A at time t and time zero, respectively, k is the rate constant.
We can use the given information to calculate the rate constant (k) of the reaction for the given half-life (t1/2) of 3.15 minutes:
t1/2 = (1 / k[A]0)
Using the percentage decrease in concentration and the given initial concentration, we can calculate the concentration of A at time t:
[A]t = [A]0 - 0.30[A]0 = 0.57126 M
Substituting the given values, we get:
3.15 min = (1 / k)(0.0818 M) / (0.0818 M - 0.57126 M)
Simplifying the equation above, we can solve for k:
k = 0.111 M^-1 min^-1
Therefore, the rate constant of the second-order reaction is 0.111 M^-1 min^-1.
For such more questions on constant
https://brainly.com/question/3159758
#SPJ11
Which structure is the Lewis structure for ammonia (NH3)?
A.
A bond line structure of a compound has N H H H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
B.
A bond line structure of a compound has H N H in the linear plane and hydrogen is branching upward, and the compound is H N (H) H.
C.
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons.
D.
A bond line structure of a compound has H N H H. The nitrogen has two dots on its top represents a lone pair of electrons.
Answers
Answer: **
H-N-H
|
H
Explanation:
Look at a periodic table to determine how many electrons you need to account for. Hydrogen (H) only has 1 electron, while Nitrogen (N) has 5. We have three Hydrogen atoms and one Nitrogen atom, so the total number of electrons will be 3 * 1 + 5 = 8 e-.
Now, place the center atom, which will be Nitrogen and place the three Hydrogens on three sides of it as above in the answer. You should use single bonds for this. Each single bond is a pair of electrons, so since we have three single bonds so far, we have accounted for 2 * 3 = 6 electrons. However, we need 2 more electrons for the total of 8. We put these electrons in as a lone pair above Nitrogen.
We check to see if everything follows the octet rule: Nitrogen has three single bonds, so that's 6 e-, as well as one lone pair, so that's another 2 e- for a total of 8 e-. Check. Now look at Hydrogen: H is the only element whose full orbital is 2 e-. Each H has a single bond with Nitrogen, so each does have 2 e-.
Thus, we know this is the correct diagram, and we are done.
Explanation:
A bond line structure of a compound has H N H in linear plane and a hydrogen is branching upward, and the compound is H N (H) H. The nitrogen has two dots at its bottom represents a lone pair of electrons. So ,the correct answer is option C.
The correct Lewis structure for ammonia (\(NH_3\)) is option C. It shows a bond line structure with three hydrogen atoms (H) bonded to a central nitrogen atom (N) in a linear plane.
One hydrogen atom branches upward from the plane. Additionally, the nitrogen atom in this structure has two dots at its bottom, indicating a lone pair of electrons. This arrangement follows the octet rule, as nitrogen has formed three covalent bonds with hydrogen, completing its valence shell. The lone pair on nitrogen gives ammonia its characteristic properties.
Thus, option C accurately represents the Lewis structure of ammonia, showing the bonding and lone pair arrangement of its atoms.
To know more about bond line structure:-
https://brainly.com/question/30639285
Given the following reaction:
2 ZnS(s) + 3 O2(g) → 2 ZnO(s) + 2 SO2(g) ΔH = -927.54 kJ
How much energy would be released if 50.0g of ZnS(s) is reacted with 30.0g of O2(g)?
Answers
Answer: -354.78 kJ of energy would be released for a given amount.
Explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass.
The equation used is:
\(\text{Number of moles}=\frac{\text{Given mass}}{\text{Molar mass}}\) ......(1)
For Zinc:
Mass of zinc = 50.0 g
Molar mass of zinc = 65.38 g/mol
Plugging values in equation 1:
\(\text{Moles of Zn}=\frac{50.0g}{65.38g/mol}=0.765mol\)
For oxygen gas:
Mass of oxygen gas = 30.0 g
Molar mass of oxygen gas = 32 g/mol
Plugging values in equation 1:
\(\text{Moles of }O_2=\frac{30.0g}{32g/mol}=0.9375mol\)
The chemical equation follows:
\(2ZnS(s)+3O_2(g)\rightarrow 2ZnO(s)+2SO_2(g);\Delta H=-927.54kJ\)
By the stoichiometry of the reaction:
If 2 moles of zinc reacts with 3 moles of oxygen gas
So, 0.765 moles of zinc will react with = \(\frac{3}{2}\times 0.765=0.51mol\) of oxygen gas
As the given amount of oxygen gas is more than the required amount. Thus, it is present in excess and is considered as an excess reagent.
Thus, Zn is considered a limiting reagent because it limits the formation of the product.
By the stoichiometry of the reaction:
If 2 moles of zinc are releasing 927.54 kJ of energy
So, 0.765 moles of zinc will react with = \(\frac{927.54}{2}\times 0.765=354.78kJ\) of energy
Hence, -354.78 kJ of energy would be released for a given amount.
The atomic number of an atom is always equal to the total number of
A. neutrons in the nucleus
B. protons in the nucleus
C. neutrons plus protons in the atom
D. protons plus electrons in the atom
Answers
Answer:
yep C is correct!!
Explanation:
Which statement is true concerning visible light and the electromagnetic spectrum?
A.
Visible light is a large portion of the electromagnetic spectrum.
B.
Visible light is a small portion of the electromagnetic spectrum.
C.
All electromagnetic radiation is considered to be visible.
Answers
Answer:
The true statement concerning visible light and electromagnetic radiation is considered to be - d. Visible light is a small portion of the electromagnetic spectrum.
The electromagnetic spectrum is the range of frequencies of electromagnetic waves or radiations. This spectrum also gives an idea about the wavelengths of electromagnetic radiations. The part of the spectrum on their higher to lower energy or frequency range from Hz to Hz are:
gamma rays
X-rays
ultraviolet radiation
visible light
infrared radiation
radio waves.
The visible light is the very small part of the electromagnetic spectrum that ranges from the to, Visible light is the light that the human eye can see.
Thus, the correct answer is- option d.
Learn more about visible light:
Explanation:
i did this
The partial pressure of F2 and a mixture of gases were the total pressure is one ATM. What is the mole fraction of F2?
Answers
The partial pressure of F2 and a mixture of gases where the total pressure is one atm. The mole fraction of F2 is 0.394736.
What is mole fraction ?
The term mole fraction is defined as the number of molecules of a component in a mixture is divided by the total number of moles in the given mixture.
Total pressure = 1 atm
= 760 torr
Then,
The partial pressure of F2 = 300 torr
The mol fraction of F2 = PF2/PT
= 300/760
= 0.394736
Thus, The partial pressure of F2 and a mixture of gases where the total pressure is one atm. The mole fraction of F2 is 0.394736.
To learn more about the mole fraction, follow the link;
https://brainly.com/question/8076655
#SPJ1
How did the Bohr model of the atom affect scientific thought?
A. Scientists accepted Bohr's model as a useful explanation
B. Scientists rejected the model because it didn't fit the data
C. Scientists accepted the model at first, but later rejected it.
D. Scientists finally agreed that Bohr's model was accurate,
Answers
Answer:
C. Scientists accepted the model at first but later rejected it.
Explanation:
Scientists accepted the model at first because it explained the hydrogen emission spectrum.
However, with the development of quantum mechanics, scientists had to modify the model (not reject it).
Electrons still had specific energies, but they no longer travelled in fixed orbits.
Instead, electrons had a probability of being found in a given region of space.
Scientists accepted the model at first but later rejected it.
How did Bohr change science?Niels Bohr change the atomic theory by realizing that the electrons did not crash into the nucleus as would be expected in classical physics. Classical physics says that opposites attract and like to repel, so the negative electrons should be attracted to the positive nucleus.
What did Bohr contribute to modern theory?In 1913, Niels Bohr proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values. Electrons move around a nucleus, but only in prescribed orbits, and If electrons jump to a lower-energy orbit, the difference is sent out as radiation.
Learn more about Niels Bohr here https://brainly.com/question/1402660
#SPJ2
If 6g of Mg completely react with excess oxygen, what volume of oxygen will be consumed?
Answers
The number of volume of the oxygen that would be consumed is 2.8 L.
What is the volume of the oxygen that would be consumed?Let us recall that what we have here is the process of the use of the stoichiometry of the reaction so as to be able to obtain the mass and the volume of the reactants that are taking place in the reaction as we are going to have in the case that is at hand.
What we now need to do so that we can be able to use the stoichiometry of the reaction would just be to write down the equation of the reaction and this is going to give us the reaction; \(2Mg(s) + O_{2} (g) ---- > 2MgO\).
In this case we know that;
Number of moles of the magnesium = 6g/24 g/mol
= 0.25 moles
Now we know that;
If 2 moles of the magnesium reacts with 1 mole of the oxygen then
0.25 moles of the magnesium would react with 0.25 * 1 /2
= 0.125 moles
Volume of oxygen that is consumed is; 0.125 moles * 22.4 L
=2.8 L
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
can you help me please

Answers
Answer:
Concentrated solutions
QUICK AND EASY, NO COMPLICATED PROBLEM!
Pennies used to be made of copper and zinc and had a mass of 3.1g. Today, pennies are made of copper-plated zinc and have a mass of 2.5g. A new penny's mass is what percent of an old penny's mass?
Answers
Answer:
81%
Explanation:
what are all of the living and nonliving things in an area called?
Answers
Barry makes a hypothesis that driving with his windows closed will increase
his gas mileage. If the gas mileage does not change, what should Barry do
next?
A. Conclude that the gas additive has gone bad
B. Assume that the hypothesis is not useful because it made a wrong
prediction
C. Change the results to show that the hypothesis is correct
D. Repeat the experiment to confirm the result
Answers
Answer: D. Repeat the experiment to confirm the results
help.
Which of the following human activities could lead to more frequent red tides?
A. Adding fertilizers to plants in your yard.
B. Oil left behind by cars driving on city roads.
C. Runoff from factories that are located near oceans.
D. All of the above.
Answers
Answer:
D
Explanation:
How many atoms of oxygen are in one molecule of water (H2O)? one two four three
Answers
Answer:
there is one atom of oxygen and two atoms of hydrogen
Explanation:
For this experiment you will need to perform a serial dilution of CO(NO3)2 solutions, meaning that you will begin with a stock solution, dilute it to make a new solution, and then use that new solution as the stock solution for the next dilution. You will start with a 0.25 M CO(NO3)2 solution. Using the values below, calculate the volume of solution and water needed at each step of the dilution.
Concentration of original solution mL of original solution required mL of water required Concentration of new solution
0.25M 0.1M
0.1M 0.05M
0.05M 0.01M
Answers
Answer:
See answer below
Explanation:
In this case, I will put the original photo of this exercise, because we are missing one data. The first picture is the original exercise.
Now, according to this, we need to make a serial dilution of CO(NO₃)₂. We don't know the volume of this solution, but we do know the total volume of the preparing solution (In the picture states that the total volume will be 10 mL).
So, we know the final volume of the solutions to be prepared, so, le'ts use the expression that will help us to solve this:
C₁V₁ = C₂V₂
Where:
C₁: Concentration of the given solution (stock)
V₁: volume required to prepare the dilluted solution
C₂; Concentration of the dilluted solution
V₂: Total volume of the dilluted solution.
Now that we know the expression to use and the meaning of each value, let's prepare the solutions:
To prepare 10 mL of 0.1 M using a 0.25 M, we will replace these values in the above expression; from there, we will solve for V₁, that value will tell us the required volume to prepare solution 2, and then, by difference we can calculate the volume of water:
Volume of water (Vw) =V₂ - V₁
Now replacing the values:
0.25V₁ = 0.1 * 10
V₁ = 1/0.25 = 4 mL
V₁ = 4 mLThis means that we need 4 mL of the stock to prepare the 0.1 M of dilluted solution, therefore, the volume of water required is:
Vw = 10 - 4
Vw = 6 mLUsing these same steps for the other two solutions we will get V1 and V2 for both of them. In this case, I will go straight to the procedure without further explanation because it's the same of this one.
For solution 2:
0.1V₁ = 0.05 * 10
V₁ = 0.5/0.1
V₁ = 5 mLVw = 10 - 5
Vw = 5 mLFinally for solution 3:
V₁ = 0.01 * 10 / 0.05
V₁ = 2 mLVw = 10 - 2 mL
Vw = 8 mLHope this helps

8. The substance made as a result of a chemical reaction is called the
A. reactant
D. product
Which ONE????
Answers
Answer:
product
Explanation:
its the result of the reaction.
5. Infer if a water molecule (H20) has two hydrogen atoms and one oxygen atom, how would you describe the make- up of a carbon dioxide molecule (CO2)?
(please help)

Answers
Answer:
The correct answer is - one carbon atom and two oxygen atoms.
Explanation:
Carbon dioxide is a gas that is present in the atmosphere of the earth. The chemical formula of carbon dioxide is CO₂. In this colorless gas, there is one molecule of carbon dioxide is made up of one atom of carbon covalently double bonded to the two atoms of the oxygens.
Thus, the make- up of a carbon dioxide molecule (CO2) includes one carbon atom and two oxygen atoms.
The K of a given reactions is 432. Is the reaction favorable or not favorable?
Answers
Answer:Favorable
Explanation:um I know That it is Favorable sorry!
A chemist measures the amount of oxygen gas produced during an experiment. She finds that of oxygen gas is produced. Calculate the number of moles of oxygen gas produced. Be sure your answer has the correct number of significant digits.
Answers
Answer:
This question is incomplete. A fragment of the question is missing. The fragment is:
She finds that 4.87 g of oxygen gas is produced
0.152 moles
Explanation:
Using the formula: mole = mass / molar mass
According to the question, a chemist measures the amount of oxygen gas produced during an experiment. Oxygen gas has the chemical formula: O2. Hence, the molar mass will be:
O2 = 16(2) = 32g/mol
If the mass of the oxygen gas to be 4.87 g of oxygen gas.
Therefore,
moles = 4.87/32
moles = 0.152mol
Is anyone good at chemistry if so Can someone help me please NO LINKS

Answers
Answer:
6.25 g
Explanation:
From the question given above, the following data were obtained:
Half-life (t½) = 68.8 years
Time (t) = 344 years
Original amount (N₀) = 200 g
Amount remaining (N) =?
Next, we shall determine the number of half-lives that has elapsed. This can be obtained as follow:
Half-life (t½) = 68.8 years
Time (t) = 344 years
Number of half-lives (n) =
n = t / t½
n = 344 / 68.8
n = 5
Thus, 5 half-lives has elapsed.
Finally, we shall determine the amount of the Uranium-232 that remains. This can be obtained as follow:
Original amount (N₀) = 200 g
Number of half-lives (n) = 5
Amount remaining (N) =?
N = 1/2ⁿ × N₀
N = 1/2⁵ × 200
N = 1/32 × 200
N = 200 / 32
N = 6.25 g
Thus, the amount of Uranium-232 that remains is 6.25 g
QUICK!!!
The specific heat of mercury is 0.138 J/g Celsius. If 452 g of mercury at 85.0 Celsius are placed in 145 g of water at 23.0 Celsius, what will be the final temperature for both the mercury and the water?
Answers
Answer: Thus the final temperature for both the mercury and the water is \(28.8^0C\)
Explanation:
The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity.
\(heat_{released}=heat_{absorbed}\)
\(Q=m\times c\times \Delta T=m\times c\times (T_{final}-T_{initial})\)
\(-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]\)
Q = heat absorbed or released
\(m_1\) = mass of mercury= 452 g
\(m_2\)= mass of water = 145 g
\(T_{final}\) = final temperature = ?
\(T_1\) = temperature of mercury = \(85.0^0C\)
\(T_2\) = temperature of water = \(23.0^0C\)
\(c_1\) = specific heat of mercury = \(0.138J/g^0C\)
\(c_2\) = specific heat of water= \(4.184J/g^0C\)
Now put all the given values in equation (1), we get
\(-[452\times 0.138\times (T_f-85.0)^0C]=145\times 4.184\times (T_f-23.0)^0C\)
\(T_f=28.8^0C\)
Thus the final temperature for both the mercury and the water is \(28.8^0C\)
How moles of Cl' are in a 45 mL of 1,4 M solution of NaCl?
Answers
Answer:
.063 moles Cl-
Explanation:
To find moles multiply molarity by volume.
1.4*.045=.063 moles Cl-
What determines how much something will change temperature?
Answers
Answer:
The heat Q transferred to cause a temperature change depends on the magnitude of the temperature change, the mass of the system, and the substance and phase involved. (a) The amount of heat transferred is directly proportional to the temperature change.
Hope it helps, BE SAFE! :3
If 35.3 mL of a Ca(OH)2 solution are needed to neutralize 24.6 mL of 0.02 M HC2H3O2 solution, what is the concentration (molarity) of the Ca(OH)2 solution?Select one:a.0.01 Mb.1.5 Mc.1.2 Md.0.007 M
Answers
Answer:
\(D:\text{ 0.007 M}\)Explanation:
Here, we want to calculate the molarity of the calcium hydroxide solution
We start by writing the equation of reaction:
\(Ca(OH)\placeholder{⬚}_2\text{ +2 CH}_3COOH\text{ }\rightarrow\text{ Ca\lparen CH}_3COO)\placeholder{⬚}_2\text{ + 2H}_2O\)Now, we proceed to write the standardization equation:
\(\frac{C_aVa}{C_bV_b}\text{ = }\frac{n_a}{n_b}\)where:
Ca is the molarity of the acid which is 0.02 M
Va is the volume of the acid which is 24.6 mL
Cb is the molarity of the base which is what we want to calculate
Vb is the volume of the base which is 35.3 mL
na is the number of moles of the acid in the balanced equation which is 2
nb is the number of moles of the base in the balanced equation which is 1
Substituting the values, we have:
\(\begin{gathered} \frac{0.02\times24.6}{C_b\times35.3}\text{ = }\frac{2}{1} \\ \\ C_b\text{ = }\frac{0.02\times24.6}{35.3\times2}\text{ = 0.007 M} \end{gathered}\)
Calculate the mass percent by volume of 34.1 g of glucose
(C.H120., MM = 180.2 g/mol) in
325 mL of solution.
Answers
Answer:
10.5%
Explanation:
The equation used to calculate mass percent by volume is:
Mass Solute (g)
Mass/Volume % = ----------------------------------- x 100%
Volume Solution (mL)
In this case, the solute is the glucose. You can plug the given values into the equation and solve.
34.1 g C₆H₁₂O₆
Mass/Volume % = ------------------------------- x 100%
325 mL soln
Mass/Volume % = 0.105 x 100%
Mass/Volume % = 10.5%
what is the concentration of a nitric acid solution if 10.0 ml of the solution is neutralized by 3.6 ml of 0.2 m naoh?
Answers
Answer:
The concentration of the nitric acid (HNO3) solution is 72 M.
Explanation:
To determine the concentration of the nitric acid solution, we can use the concept of stoichiometry and the equation of the neutralization reaction between nitric acid (HNO3) and sodium hydroxide (NaOH):
HNO3 + NaOH → NaNO3 + H2O
The balanced equation shows that the molar ratio between HNO3 and NaOH is 1:1. This means that 1 mole of HNO3 reacts with 1 mole of NaOH.
Given:
Volume of HNO3 solution = 10.0 ml
Volume of NaOH solution = 3.6 ml
Molarity of NaOH solution = 0.2 M
To find the concentration of the HNO3 solution, we need to calculate the number of moles of NaOH used in the neutralization reaction:
moles of NaOH = volume of NaOH solution * molarity of NaOH solution
= 3.6 ml * 0.2 M
= 0.72 mmol (millimoles)
Since the molar ratio between HNO3 and NaOH is 1:1, the number of moles of HNO3 in the solution is also 0.72 mmol.
Now, we can calculate the concentration of the HNO3 solution using the formula:
concentration (in M) = moles of solute / volume of solution (in L)
concentration = 0.72 mmol / 0.010 L
= 72 mmol/L
= 72 M
Therefore, the concentration of the nitric acid (HNO3) solution is 72 M.
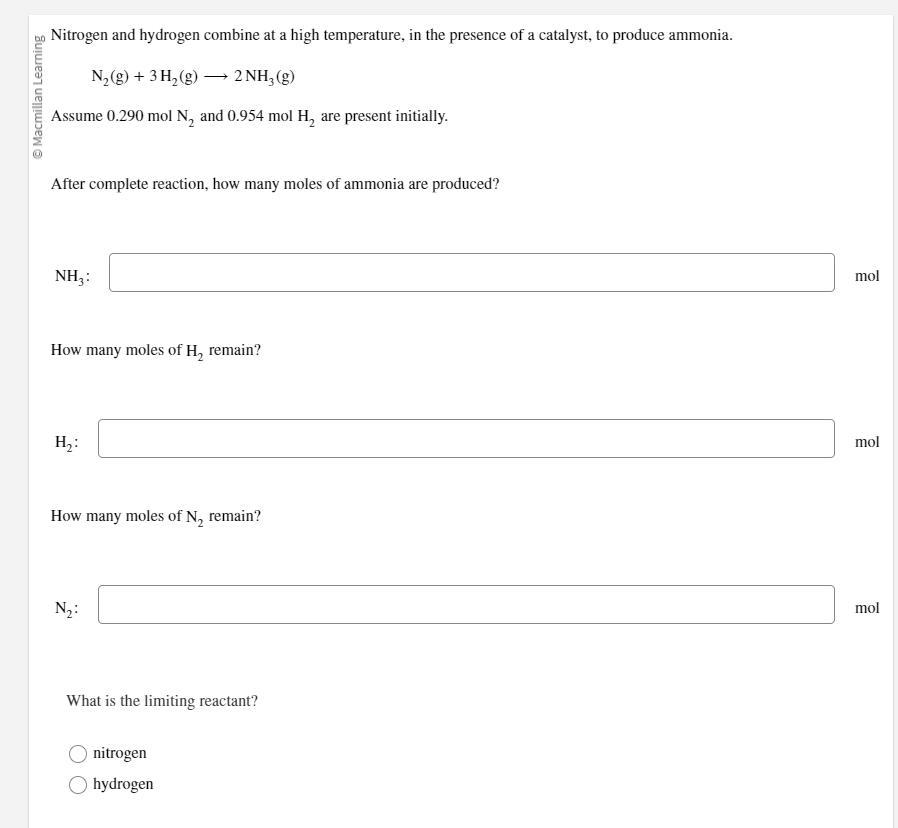
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
Answers
Answer:
17 reactions
Explanation:
Zeros between nonzero digits are significant
Answers
Answer:
Explanation:
If a zero is found between significant digits, it is significant
Wine goes bad soon after opening because Ethanol (CH3CH2OH) dissolved in it reacts with oxygen (O2) gas to form water and Aqueous Acetic acid (CH3COOH) The main ingredient of vinegar. Calculate the moles of water produced by the reaction of 0.060 mol of oxygen
Answers
Answer:
\(0.060molH_2O\)
Explanation:
Hello there!
In this case, since the chemical reaction between ethanol and oxygen to water and acetic acid is:
\(CH_3CH_2OH+O_2\rightarrow CH_3COOH+H_2O\)
Thus, since there is a 1:1 mole ratio between oxygen and water, the produced moles of water are calculated down below:
\(0.060molO_2*\frac{1molH_2O}{1molO_2}=0.060molH_2O\)
Best regards!