a. Draw the Fischer projection (standard orientation) for L-Glucose and D-Glucose.b. What is the isomeric relationships for the two structures you drew?c. What do the D and L designations mean?d. Label each chirality center on the structures you drew either R or S. e. What can you say about the orientation of the OH group at each chirality center in a Fischer projection for a sugar in standard orientation (highest oxidizedgroup on top) and the R and S designation?
Answers
a. In the Fischer projection of L-Glucose, the hydroxyl (OH) groups are oriented as follows: right, left, right, left, and left. For D-Glucose, the orientation is: right, left, right, left, and right.
b. The isomeric relationship between L-Glucose and D-Glucose is enantiomers, as they are non-superimposable mirror images of each other.
c. The D and L designations indicate the stereochemistry of the chiral carbon furthest from the highest oxidized group (typically an aldehyde or ketone) in a sugar molecule. If the hydroxyl group on this carbon is on the right in a Fischer projection, it's designated as D; if on the left, it's designated as L.
d. In L-Glucose, the chirality centers are C2 (S), C3 (R), C4 (S), and C5 (R). In D-Glucose, the chirality centers are: C2 (R), C3 (S), C4 (S), and C5 (R).
e. In a Fischer projection for sugar in standard orientation, if the OH group at a chirality center is on the right, it is designated as R. If the OH group is on the left, it is designated as S. This helps determine the stereochemistry of the sugar molecule.
To know something about the Fischer projection, click below.
https://brainly.com/question/31607590
#SPJ11
Related Questions
Can someone please explain what orbits are? Like for example, how many orbits an element has
Answers
Answer: There are many possibilities for atomic orbits.
Explanation: In chemistry orbits, or orbitals, are the areas that electrons move around the nucleus of an atom. Think like the solar system.
There are three levels of orbitals (p,d, and f).
That should get you started. Use p, d and d described in your book to find out how many orbitals an atom has.
Which is an empirical formula?
1.
H202
2.
H20
3.
C₂H₂
4.
C3H6
Answers
Answer:
c2h2 is it..........
Explanation:
lol
As a result of this process, the proportions of oxygen and carbon dioxide in
air breathed in and air breathed out change.
Which one of the statements is true? Tick the correct box. [1]
- Air breathed out has less carbon dioxide and more oxygen than air breathed in.
- Air breathed out has less carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and more oxygen than air breathed in.
Answers
Answer:
the third one
Explanation:
When you breathe in, you inhale oxygen and exhale carbon dioxide
How can we distinguish between ethylene and acetylene chemically?
Answers
Answer:
The key difference between acetylene and ethylene is that acetylene has a triple bond between two carbon atoms whereas ethylene has a double bond between two carbon atoms. The names acetylene and ethylene sound similar, but they are different hydrocarbon compounds.Both ethylene and acetylene are hydrocarbons but the former has a carbon to carbon double bond while the latter has a carbon to carbon triple bond.
Ethylene and acetyleneBoth organic compounds are referred to as hydrocarbons.
They are also both unsaturated.
Ethylene contains a C-C double bond and belongs to the alkene group.
Acetylene contains a C-C triple bond and belongs to the alkyne group.
More on hydrocarbons can be found here: https://brainly.com/question/17578846
What happens to the other ions that are not attracted to the electrodes?
Answers
Ions can be made by single element or covalently bonded group of elements. The covalently bonded group of elements is called polyatomic ions or polyatomic atoms. Therefore, the other ions that are not attracted to the electrodes form salt.
What is Ions?Any species that contain charge whether it is positive charge or negative charge is called ions. The example of polyatomic ions are sulfate, phosphate, nitrate etc.
Cation is the species that loose electron and attain positive charge while anion is a species which gain electron and attains negative charge so when anion and cation combine in fixed ration the the overall charge of the molecule is zero that is molecule is neutral, the charge over cation and anion is also called oxidation state.
Positive ions are attracted towards negative electrode and negative ions are attracted toward positive electrodes. the ions which are not attracted to either of the electrode, these form salt with the other ion remaining in the electrolyte.
Therefore, the other ions that are not attracted to the electrodes form salt.
To learn more about ions, here:
https://brainly.com/question/13692734
#SPJ1
what is conduction,convection,and radiation in science brainiest nice and great answer report wrong answer
Answers
If you put 10.0mL of a in one balance pan . How much mass of b would you need in the other pan to make it balanced
Answers
To balance 10.0 mL of substance a, we would need 6.67 g of substance b.
How did we arrive at the value?To determine how much mass of substance b would be needed to balance 10.0mL of substance a on the other pan, we need to know the density of both substances.
If we know the density of substance a and the volume of substance b, we can calculate the mass of substance b needed to balance the system.
For example, if we assume that substance a has a density of 1.0 g/mL, and we want to balance it with substance b which has a density of 1.5 g/mL, we can use the following formula:
mass of substance b = (density of substance a) x (volume of substance a) / (density of substance b)
Substituting the values given, we get:
mass of substance b = (1.0 g/mL) x (10.0 mL) / (1.5 g/mL)
mass of substance b = 6.67 g
Therefore, to balance 10.0 mL of substance a, we would need 6.67 g of substance b.
learn more about mass of a substance: https://brainly.com/question/837939
#SPJ1
What is the atomic mass of w
Answers
Answer:
183.84 u
Explanation:
W (also known as "Tungsten") atomic number is 74 and atomic mass is 183.84 u
How much energy (kilojoules) is required to melt 25 moles of ice?
Answers
The energy is 150 kJ
What is the latent heat of fusion?
The latent heat of fusion is the amount of energy required to change the state of a substance from a solid to a liquid, or vice versa, without changing its temperature. Specifically, it is the amount of heat energy that must be added to a substance to overcome the attractive forces between its molecules and break them free from their fixed positions in the solid lattice structure.
We know that;
H = mL
m = mass of the ice
L = latent heat of fusion in J/mol
Thus;
H = 25 mol * 5.99 KJ/ mol
H = 150 kJ
Learn more about latent heat:https://brainly.com/question/28044951
#SPJ1
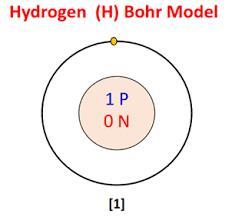
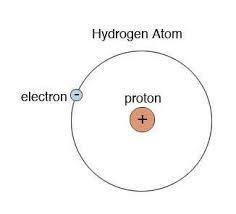
Draw the Bohr model of 2.2 Define the term isotopes 2.3 Draw the electron configuration a of an anion hydrogen atom which is a Lewis base 2.4 Given the following data calculate the average atomic mass of Hydrogen
1
H occurrence 99.98%
2
1
H occurrence 0.0156%
3
1
H occurrence 0.0044% 2.5 How is hydrogen related to alkali metals and halogens? 2.6 Use Balmer's equation the two largest wavelengths of the Lyman series. 2.7 Calculate energy required for the photon to ionize a ground state hydrogen atom (From ni=1 to nf=[infinity] ). Use RH=1.097×10
7
m
−1
and h=6,63×10
−34
J. /21/
Answers
The electron is in the first energy level (n = 1) closest to the nucleus. The electron configuration of the anion hydrogen atom is 1s² 2s² 2p⁶, similar to the electron configuration of helium. The exact average atomic mass of hydrogen is 1.000232 g/mol. Two largest wavelengths of the Lyman series are 0 and 4RH/36. Energy required to ionize a ground state hydrogen atom from n(i) = 1 to n(f) = ∞ is RH.
2.2: The Bohr model of hydrogen consists of a nucleus at the center (proton) and an electron orbiting the nucleus in a circular orbit. The electron is in the first energy level (n = 1) closest to the nucleus. The figure is given below.
2.3: The electron configuration of an anion hydrogen atom, which is a Lewis base, means that it has gained an extra electron. The electron configuration of the anion hydrogen atom is 1s² 2s² 2p⁶, similar to the electron configuration of helium.
2.4: To calculate the average atomic mass of hydrogen:
Average Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + (Mass of Isotope 3 × Abundance of Isotope 3)
Given the data:
1H occurrence: 99.98%
2H occurrence: 0.0156%
3H occurrence: 0.0044%
Substituting the values into the formula:
Average Atomic Mass = (1 g/mol × 0.9998) + (2 g/mol × 0.000156) + (3 g/mol × 0.000044)
Calculating the result:
Average Atomic Mass = 0.9998 g/mol + 0.000312 g/mol + 0.000132 g/mol
Average Atomic Mass = 1.000232 g/mol
Therefore, the exact average atomic mass of hydrogen is 1.000232 g/mol.
2.6: Using Balmer's equation, let's calculate the two largest wavelengths of the Lyman series.
For n = 2:
1/λ = RH[(1/4) - (1/n²)]
1/λ = RH[(1/4) - (1/2²)]
1/λ = RH[(1/4) - 1/4]
1/λ = 0
For n = 3:
1/λ = RH[(1/4) - (1/n²)]
1/λ = RH[(1/4) - (1/3²)]
1/λ = RH[(1/4) - 1/9]
1/λ = 8RH/36 - 4RH/36
1/λ = 4RH/36
Therefore, the two largest wavelengths of the Lyman series are 0 and 4RH/36.
2.7: Let's calculate the energy required to ionize a ground state hydrogen atom from n(i) = 1 to n(f) = ∞.
E = RH[(1/ni²) - (1/nf²)]
E = RH[(1/1²) - (1/∞²)]
E = RH[1 - 0]
E = RH
Therefore, the exact energy required to ionize a ground state hydrogen atom from n(i) = 1 to n(f) = ∞ is RH.
To know more about hydrogen :
https://brainly.com/question/33741818
#SPJ4
A chemical equation is shown:
C2H4 + O2 → CO2 + H2O
What are the reactant(s) of this reaction? Choose all that apply
02
O C₂H4
O CO2
O H20
Answers
Explanation:
in the given chemical equation
C2H4 + O2 → CO2 + H2O
The reactants of this reaction IS
C2H4 and O2
Heterogéneo de tres fases y un componente.
Answers
Sólido (hielo)
Líquido (agua)
Gaseoso (vapor)
needed this done forever ago pls help

Answers
The following conversions from moles to liters and vice versa are:
112 L H₂O224 L O₂22.3 moles H₂1.91 moles Br₂4.46 moles He179.2 L Cl₂1 mole44.6 moles Ne246.4 L Cl₂2.12 molesHow to convert?5 moles H₂O x (22.4 L H₂O/1 mole H₂O) = 112 L H₂O
10 mole O₂ x (22.4 L O₂/1 mole O₂) = 224 L O₂
500 L H₂ x (1 mole H₂/22.4 L H₂) = 22.3 moles H₂
85 L Br₂ x (1 mole Br₂/44.6 L Br₂) = 1.91 moles Br₂
100 L He x (1 mole He/22.4 L He) = 4.46 moles He
8 moles Cl₂ x (22.4 L Cl₂/1 mole Cl₂) = 179.2 L Cl₂
22.4 L x (1 mole/22.4 L) = 1 mole
1000 L Ne x (1 mole Ne/22.4 L Ne) = 44.6 moles Ne
11 moles Cl₂ x (22.4 L Cl₂/1 mole Cl₂) = 246.4 L Cl₂
The number of moles of gas in a given volume can be calculated using the ideal gas law: PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature in Kelvin.
Assuming standard temperature and pressure (STP), which is 0°C (273.15 K) and 1 atm, respectively, the calculation would be:
PV = nRT
(1 atm) (55.5 L) = n (0.0821 L•atm/mol•K) (273.15 K)
n = (1 atm) (55.5 L) / (0.0821 L•atm/mol•K) (273.15 K)
n = 2.12 mol
Therefore, the balloon contains approximately 2.12 moles of gas.
Find out more on STP here: https://brainly.com/question/27100414
#SPJ1
Plate movement is thought to be the result of what
Answers
Answer: Plate movement is thought to be driven by a combination of the motion of the seafloor away from spreading ridges due to variations in topography and density changes in the crust.
Hope this helps
Identify the Bronsted acids and bases in the following equation (multiple choice)

Answers
Reactions according to Bronsted are those in which there is an exchange of H+ ions. The Bronsted acid will be the substance that donates H+ ions and the base will be the compound that receives them.
As a result we will have a conjugated acid and a conjugated base. The conjugate acid will be the former base that just received the H+ ions. The conjugate base will be the former acid that donated its H+ ions.
Now let's look at the reaction. On the reactants side the compound that can donate H+ ions will be HSO3- and the compound CN- will be the one that receives them. Therefore, HSO3 will be the Bronsted acid (A) and CN will be the base (B).
HSO3 ---> A
CN -----> B
Now let's look at the product side. The compound that received the H+ ions will be the compound HCN, so this will be the conjugate acid (Ac). And the compound that lost its H+ ions is SO3, so it will be the conjugate base (Bc).
HCN --->Ac
SO3 ---->Bc
Comparing the analysis that we did with the reaction we will have that:
\(\begin{gathered} HS_{}O^-_3+CN^-\rightleftarrows HCN+SO^{2-}_3 \\ A+B\rightleftarrows Ac+Bc \end{gathered}\)So, the answer will be option number 5
In your own words, explain why we have seasons on Earth. Why do some places have all 4 seasons and others seem to only have 1 or 2?
Answers
some places have 4 seasons because they are in the northern hemisphere, which is in the sweet spot of the sun's rays that give us each season. other places only have 1 or 2 because they are not in the northern hemisphere.
An ethylene glycol solution contains 30.8 g of ethylene glycol (C2H6O2) in 96.6 mL of water. (Assume a density of 1.00 g/mL for water.) Determine the freezing point of the solution. Determine the boiling point of the solution
Answers
The freezing point of the solution is -11.8 °C.
The boiling point of the solution is 103.31 °C.
To determine the freezing point of the solution, we can use the equation:
ΔTf = Kf * m
where:
ΔTf is the freezing point depression,
Kf is the cryoscopic constant (for water, Kf = 1.86 °C/m),
m is the molality of the solution (moles of solute per kilogram of solvent).
First, let's calculate the molality (m) of the solution:
Molar mass of ethylene glycol (C2H6O2):
C = 12.01 g/mol
H = 1.01 g/mol (x 6) = 6.06 g/mol
O = 16.00 g/mol (x 2) = 32.00 g/mol
Total molar mass = 12.01 g/mol + 6.06 g/mol + 32.00 g/mol = 50.07 g/mol
Number of moles of ethylene glycol (C2H6O2) = mass / molar mass
Number of moles = 30.8 g / 50.07 g/mol = 0.615 mol
Mass of water = volume x density = 96.6 mL x 1.00 g/mL = 96.6 g
Now, let's calculate the molality:
Molality (m) = moles of solute / mass of solvent (in kg)
Molality = 0.615 mol / 0.0966 kg = 6.36 mol/kg
Now we can calculate the freezing point depression (ΔTf):
ΔTf = Kf * m
ΔTf = 1.86 °C/m * 6.36 mol/kg = 11.8 °C
To find the freezing point of the solution, subtract the freezing point depression from the freezing point of pure water (0 °C):
Freezing point = 0 °C - 11.8 °C = -11.8 °C
To determine the boiling point of the solution, we can use the equation:
ΔTb = Kb * m
where:
ΔTb is the boiling point elevation,
Kb is the ebullioscopic constant (for water, Kb = 0.52 °C/m),
m is the molality of the solution (same value as calculated before: 6.36 mol/kg).
ΔTb = 0.52 °C/m * 6.36 mol/kg = 3.31 °C
To find the boiling point of the solution, add the boiling point elevation to the boiling point of pure water (100 °C):
Boiling point = 100 °C + 3.31 °C = 103.31 °C
To know more about ethylene glycol
https://brainly.com/question/32452413
#SPJ11
what is the cathode and anode of k2so4
Answers
O 2
Here,
Electrolysis of H
2
O takes place:
2H
2
O→O
2
+4H
⊕
+4e
−
What is the mass of 0.928 moles of Ti(SO3)2
Answers
1) You know the number of moles, you can easily work out the molar mass of Ti(SO3)2 (titanium sulfite), but you don't know the actual mass
2) By adding the mass of the atoms that make up titanium sulfite, you should get something like 207.9934 g/mol
3) To find the actual mass, you times the molar mass and the moles together
Final Answer = 193g
______ are the product or material stream in a distillation column that boils at the lowest temperature and that comes off the top of a column.
Answers
The product or material stream in a distillation column that boils at the lowest temperature and comes off the top of the column is known as the overhead product.
In a distillation column, the separation of different components in a mixture is achieved by exploiting differences in their boiling points. The column is designed to have a temperature gradient, with higher temperatures at the bottom and lower temperatures at the top. As the mixture is heated, the components with lower boiling points vaporize first and rise up the column.
The overhead product refers to the stream of vaporized components that reach the top of the column and are collected from there. These components have the lowest boiling points among the mixture and are therefore separated and removed as the overhead product.
To learn more about distillation, click here:
brainly.com/question/31829945
#SPJ11
what volume of water is needed to dissolve 2.70 grams of n2 at 25 oc under a pressure of 4.46 atm? kh for n2
Answers
2.70 grammes of N₂ must dissolve in 30.5 litres of water at 25 degrees Celsius and 4.46 atm of pressure.
The solubility of N₂ in water depends on the temperature and pressure. To determine the volume of water needed to dissolve 2.70 grams of N₂ at 25 °C and 4.46 atm, we need to use the Henry's law equation, which relates the solubility of a gas in a liquid to its partial pressure:
C = kH x P
where C is the concentration of the gas in the liquid, kH is the Henry's law constant for the gas, and P is the partial pressure of the gas above the liquid.
The Henry's law constant for N₂ in water at 25 °C is 7.07 x 10⁻⁴ M/atm.
First, we need to convert the mass of N₂ to moles using its molar mass:
moles of N₂ = 2.70 g / 28.02 g/mol = 0.0963 moles
Next, we can use Henry's law equation to find the concentration of N₂ in water:
C = kH x P = (7.07 x 10⁻⁴ M/atm) x (4.46 atm) = 3.16 x 10⁻³ M
Finally, we can use the definition of concentration (C = moles of solute / volume of solvent) to solve for the volume of water needed:
Volume of water = moles of solute / concentration = 0.0963 moles / 3.16 x 10⁻³ M = 30.5 L
Therefore, 30.5 liters of water are needed to dissolve 2.70 grams of N₂ at 25 °C under a pressure of 4.46 atm.
To learn more about volume refer to:
brainly.com/question/14710169
#SPJ4
During an experiment, solid iodine was placed in a sealed container. The container was gradually heated and purple-colored vapors of iodine formed were observed. Describe this system when it reaches phase equilibrium. (10 points)
Answers
Answer:
See explanation
Explanation:
For a chemical system in a state of dynamic equilibrium, the rate of forward reaction is equal to the rate of reverse reaction.
For this system under consideration;
I2(s)⇄I2(g)
When we heat the container, solid iodine is converted into purple coloured iodine vapour.
When equilibrium is achieved in the system, there will be no net change in the amount of solid iodine and iodine vapour present in the system since the rate of forward and reverse reactions are equal for a system in a state of equilibrium.
When 8.0 moles of chromium react, how many moles of chromium (lll) oxide are produced?
Answers
Moles of chromium (lll) oxide produced : 4 moles
Further explanationThe reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
4Cr+3O₂⇒2Cr₂O₃
moles of Cr=8 moles
From the equation, mol ratio Cr : Cr₂O₃ = 4 : 2, so mol Cr₂O₃ :
\(\tt \dfrac{2}{4}\times 8=4~moles\)
Oxygen reacts with iron to produce rust and with hydrogen to produce water. Which statement describes both reactions?
1.A different mixture is formed in each case.
2.A different solution is formed in each case.
3.Both a change of state and of elements is involved.
4.New molecules are formed but the same elements exist.
Answers
Answer:
3
Explanation:
beacuses im right
Calculate each of the following quantities:
(c) Amount (mol) of solute in 145.6 L of 0.850 M sodium cyanide
Answers
Amount (mol) of solute in 145.6 L of 0.850 M sodium cyanide
What is molarity?Molar concentration (also known as molarity, quantity concentration, or substance concentration) is a measure of the concentration of a chemical species in a solution, specifically of a solute, in terms of amount of substance per unit volume of solution. The most often used unit for molarity in chemistry is the number of moles per liter, denoted by the unit symbol mol/L or mol/dm3 in SI units. A solution with a concentration of 1 mol/L is referred to as 1 molar, or 1 M.
To learn more about molarity visit:
https://brainly.com/question/8732513
#SPJ4
What process is this balanced equation? I + I -> I 2
Answers
The process is this balanced equation is I₂ + I⁻ = I⁻₃. Molecules of elemental iodine are consist of two atoms of iodine.
What is iodine ?Iodine is highly stable halogen. The chemical element iodine have an atomic number 53. The symbol of iodine is "I". It is present in halogen group.
An iodine atom formed by gaining an electron due to its high electron affinity. When it accepts an electron it will become iodide ion (I⁻).
Thus, The process is this balanced equation is I₂ + I⁻ = I⁻₃. Molecules of elemental iodine are consist of two atoms of iodine.
To learn more about an iodine, follow the link;
https://brainly.com/question/16867213
#SPJ1
PLEASE HELP ME.
Where on figure 26 is a hurricane located? How does the air pressure near its center compare the air pressure in Tampa Bay, Florida

Answers
Answer:
its located on the east of Florida
Explanation:
loo at the map, and youll see it
How many electron groups exist at the central c atom in a molecule of methane ch4?.
Answers
There are four electron groups around the central carbon atom in a molecule of methane (CH4). These groups are made up of a single carbon atom bonded to four hydrogen atoms. The four electron groups are arranged in a tetrahedral shape around the carbon atom.
The molecule of methane is tetrahedral because it consists of four carbon atoms bonded to each other in a tetrahedral shape. The tetrahedral shape is the most stable shape for a molecule of methane because it minimizes the amount of strain on the bonds between the carbon atoms. The four carbon atoms in methane are arranged in a symmetrical fashion, which allows the molecule to rotate freely about the central carbon atom. This freedom of rotation allows the molecule to adopt different shapes, which is why methane is a gas at room temperature.
Learn more about methane at : https://brainly.com/question/12645626
#SPJ4
at very low temperatures oxygen, o2, freezes and forms a crystalline solid. which best describes these crystals? (a) ionic (b) covalent network (c) metallic (d) amorphous (e) molecular crystals
Answers
The best description for the crystals formed when oxygen freezes at very low temperatures is (e) molecular crystals.
This is because oxygen is a diatomic molecule, meaning it consists of two atoms that are held together by a covalent bond. When oxygen freezes, the molecules arrange themselves in a repeating pattern that forms a solid structure. This structure is held together by intermolecular forces, such as van der Waals forces, rather than by chemical bonds, which is why it is classified as a molecular crystal. Ionic, covalent network, metallic, and amorphous crystals have different types of bonding and structures, which do not apply to the formation of oxygen crystals.
The best description for crystals formed by oxygen (O2) when it freezes at very low temperatures is (e) molecular crystals. Molecular crystals are composed of molecules held together by weak intermolecular forces, such as van der Waals forces or hydrogen bonds. In the case of oxygen, the O2 molecules are covalently bonded within the molecule, but the interactions between the molecules in the crystal are not covalent, making molecular crystals the appropriate classification.
To know about crystals :
https://brainly.com/question/32130991
#SPJ11
Ah plz help plz! im stuck on this sadly

Answers
Answer:
water should be the answer because its one of the 4 elements and air