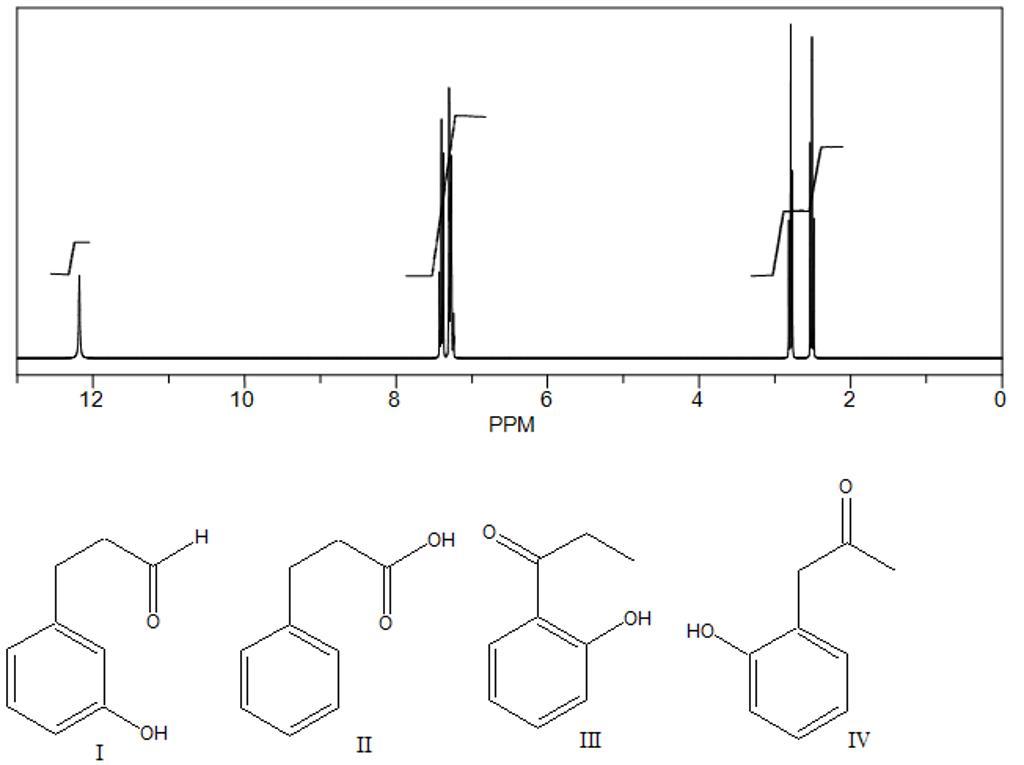
Answers
structure II is correct answer
in NMR peak spectrum at 12 ppm is only for the carboxylic acid peak
in given options there is only one option with carboxylic acid functional group .therefore the answer is structure 2 is correct answer
Structures are compatible with this spectrum since no response would occur in the absence of rotation. The change in light intensity with regard to wavelength or frequency is referred to as a "spectrum." While a spectroscope is used to see spectra visually, a spectrograph is a device used to record or map spectrum.
Options and reference diagram are provided in below the solution.
Learn more about spectrum from here:
https://brainly.com/question/21134950
#SPJ4
Related Questions
how to calculate theoretical yield
Answers
Answer:
I'm not really sure how but here's the formula?

To calculate theoretical yield first check chemical equations are balanced. Calculate the mole ratios of the reactants and products, Find the theoretical yield of the reaction.
Percent Yield = Mass of Actual Yield / Mass of Theoretical Yield x 100 percent.
What is theoretical yield ?The theoretical yield is the quantity of product that stoichiometry predicts will be produced, whereas the actual yield is the amount that is actually produced.
The yield of a reaction is used to represent how much of a product is produced from that reaction.
Thus, Divide the ratio by the limiting reactant's molecular weight. The answer is the theoretical yield of the desired product in moles.
To learn more about the theoretical yield, follow the link;
https://brainly.com/question/14966377
#SPJ1
FeCl3 + NaOH → Fe(OH)3 + NaCl
Answers
\(\qquad \qquad\huge \underline{\boxed{\sf Answer}}\)
Here's the solution ~
The required balanced equation is :\(\qquad \sf FeCl_3 + 3 \: NaOH \dashrightarrow Fe(OH)_3 + 3 \: NaCl\)
Reactant Side : Iron (Fe) = 1Sodium (Na) = 3Oxygen (O) = 3 Hydrogen (H) = 3Chlorine (Cl) = 3Product side : Iron (Fe) = 1Sodium (Na) = 3Oxygen (O) = 3 Hydrogen (H) = 3Chlorine (Cl) = 3You are out hiking on a cold snowy day. You put on your battery-heated socks. In which direction is the thermal energy flowing?
There is no thermal energy in this scenario.
Thermal energy is moving from the air to your socks
Thermal energy is moving from your feet to your socks
Thermal energy is moving from your socks to your feet
Answers
The correct answer is that thermal energy is moving from your feet to your socks. The battery-heated socks work by using the heat generated by your body to warm your feet in cold weather.
The body produces heat, which is converted into thermal energy, and moves from your feet to the socks.
In order to warm your feet and make them more comfortable in cold weather, the socks use thermal energy. The thermal energy is only transferred in one direction, from your feet to the socks.
No more energy is produced because the battery-heated socks utilise your body's thermal energy to keep your feet warm.
To learn more about thermal energy visit:
https://brainly.com/question/19666326
#SPJ1
what’s is the answer?

Answers
The energy of the photon of light can be obtained as 6.27 * 10^-20 J.
What is the energy of the photon?We know that a photon has to to do with a particular unit of light. We know that light can be said to be composed of very tiny corpuscles and these corpuscles of light is what we call the photon of the light.
We can be able to us the equation that is derived by Max Plank to be able to get the value of the energy of the photon of light. Now we know that a photon of light can have an energy that is able to be obtained by;
E = hf
h = Plank's constant
f = Frequency
Then;
E = 6.6 * 10^-34 Js * 9.5 * 10^13 Hz
= 6.27 * 10^-20 J
Thus as we can see from the parameters in the question, the energy of the photon is 6.27 * 10^-20 J.
Learn more about energy of the photon:https://brainly.com/question/2393994
#SPJ1
Weathering refers to the effects of exposure to A) insults B)pressure C)weather
Answers
c. Weather
Explanation:
Weathering process in genealogically long periods when the surface area has exposed, depends mainly on the climate of the region.
A dramatic classroom demonstration involves cooling a balloon from room temperature (293 K ) to liquid nitrogen temperature (77 K). If the initial volume of the balloon is 2.6 L , what will its volume be after it cools
Answers
The volume of the balloon after it cools is 0.68 L.
Charles law states that the volume of a gas is directly proportional to its temperature at constant pressure.
It is given by:
V ∝ T
V/T = constant
Therefore:
\(\frac{V_1}{T_1}=\frac{V_2}{T_2} \\\\V_1=2.6\ L,T_1=293\ K,T_2=77\ K:\\\\\frac{2.6}{293}=\frac{V_2}{77} \\\\V_2=\frac{2.6}{293}*77\\\\V_2=0.68\ L\)
Hence the volume of the balloon after it cools is 0.68 L.
Find out more at: https://brainly.com/question/16927784
Moles of water that were produced from the com combustion

Answers
The chemical of hydrogen has 0.0426 moles per y.
What is a combustion reaction equation example?A compound combines with an oxidising element, such as oxygen, in a complete combustion reaction, and the products are compounds of each element in the fuel and the oxidising element. This reaction is exothermic. For instance, Carbon dioxide(g) + 2water(g) + heat + light = Methane(g) + 2Oxygen(g).
Molar mass of water (Water) = 2(1.008 g/mol) + 1(15.999 g/mol) = 18.015 g/mol
moles of water = mass of water collected / molar mass of water
moles of water = 0.384 g / 18.015 g/mol
moles of water = 0.0213 mol
Therefore, 0.0213 mol of water were produced from the combustion of the compound.
To determine the moles of hydrogen in the compound, we need to use the balanced chemical equation for the combustion of hydrocarbons:
(x + y/4 - z/2)Oxygen → xCarbon dioxide + y/2water
From the equation, we can see that each mole of water produced in the combustion reaction corresponds to y/2 moles of hydrogen in the original compound.
Since we now know that 0.0213 mol of water were produced, we can calculate the moles of hydrogen in the compound:
moles of hydrogen = (2 mol of water / y mol of hydrogen) x 0.0213 mol of water
moles of hydrogen = 0.0426 mol / y
To know more about hydrogen visit:-
https://brainly.com/question/28937951
#SPJ9
Which most likely indicates a chemical change has occurred?
a solid substance becoming larger
a solid melting and becoming a liquid
a green liquid becoming a red liquid
a liquid freezing and becoming a solid
Answers
Answer:
A green liquid becoming a red liquid.
Explanation:
If the color change is unexpected it is a chemical change.
(If 2 clear liquids make a black, red, green, ect color it is a chemical change)
no normal color mix makes green turn red.
What is the molecular weight of (NH4)2SO4
Answers
The molecular weight of \((NH_4)_2SO_4\) would be 132 g/mol.
Calculation of molecular weightsThe molecular weight, otherwise known as the molar mass of substances is the sum of the molar weights of individual atoms that make up the substance.
\((NH_4)_2SO_4\) is made up of 2 atoms of N, 8 atoms of H, 1 atom of S, and 4 atoms of O.
Molar weight of N is 14 g/molMolar weight of H is 1 g/molMolar weight of S is 32 g/molMolar weight of O is 16 g/molThus, the molecular weight of \((NH_4)_2SO_4\) = (14x2) + (1x8) + 32 + (16x4)
= 132 g/mol
In other words, the molecular weight of \((NH_4)_2SO_4\) is 132 g/mol.
More on molecular weights can be found here: https://brainly.com/question/14507491
#SPJ1
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
Is atomic radius a periodic property of atoms??
Answers
Identify the limiting reactant in the reaction of methane (CH4) and carbon tetrachloride to form CH2Cl2, if 2.96 g of CH4 and 32.0 g of CCl4 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete.
Answers
Answer:
d
Explanation:
yes
Based on amino acid metabolism in mammals, classify the descriptions and amino acids is ketogenic or glucogenic. a. serine b. aspartate c. leucine d. converted to citric acid cycle intermediates e. converted to pyruvate f. converted to acetyl COA g. converted to fatty acids1) ketogenic2) glucogenic
Answers
Answer:
a. Serine is converted to pyruvate. It is glucogenic.
b. Aspartate is converted citric acid cycle intermediates. It is glucogenic.
c. Leucine is converted to AcetylCoA. It is ketogenic.
Explanation:
The degradation of amino acids usually account for a significant amount of energy production by the human body. The carbon skeletons of amino acids after deamination are either channelled to gluconeogenesis, ketogenesis or are completely oxidized to carbon dioxide and water.
The amino acids that are channelled to gluconeogenesis are said to be glucogenic. The glucogenic amino acids are those that are either degraded to ∝-ketoglutarate, succinylCoA, fumarate or oxaloacetate which are citric acid cycle intermediates that can be converted to glucose and glycogen. Also, amino acids that are degraded to pyruvate are glucogenic as pyruvate can be converted to glucose via oxaloacetate. Examples of these amino acids are serine which is converted to pyruvate and aspartate which is converted to the citric acid cycle intermediate, oxaloacetate.
On the other hand, amino acids that are channelled to ketogenesis are said to be ketogenic. The ketogenic amino acids are degraded to either acetylCoA or acetoacetylCoA. AcetylCoA and acetoacetylCoA are used in the formation of key one bodies. An example of a ketogenic amino acid is leucine which is converted both to AcetylCoA and acetoacetylCoA.
QUESTION 7
The electron configuration of an atom is 1s²2s22p63s23p3. The atomic number of the atom is
O 11
03
O 15
O 5
Answers
We can write down the electron configuration of bromine using the periodic table's building pieces.
What is Electron Configuration?The amount of electrons in each sublevel and energy level of the ground-state atom is displayed in the electron configuration of an atom. Start at the nucleus and add electrons one at a time until the total number of electrons equals the total number of protons in the nucleus to establish the electron configuration of a specific atom.
The lowest energy sublevel is awarded to each additional electron. The 1s sublevel will be the first to be filled, followed by the 2s and 2p sublevels, the 3s and 3p, the 4s and 3d, and so on. It may be crucial to understand how the electrons in an atom's partially filled sublevel are allocated throughout the orbitals.
To know more about electron configuration, visit:
https://brainly.com/question/26084288
#SPJ1
what item is made from a synthetic material
Answers
Synthetic materials are made from natural resources and can be used for many purposes.
What is synthetic material ?Synthetic materials are made by chemically changing the starting substances to create a material with different characteristics. Some examples of synthetic materials are plastics, medicines, and new fuels.
A synthetic substance may be chemically identical to a naturally-occurring substance or may be different.
Item is made from a synthetic material :
Plastic bagPlastic bottleDisposable diaperSynthetic fiber/cloth (polyester, nylon, or rayon)KevlarArtificial sweetenerSynthetic fuel (Synfuel)Synthetic rubberChloroquine (Malaria drug)Taxol(Cancer drug)Physostigmine (Glaucoma drug)Aspirin
Learn more about synthetic material here ;
https://brainly.in/question/41964471
#SPJ1
At 25 °C, only 0.0510 mol of the generic salt AB is soluble in 1.00 L of water.
What is the sp of the salt at 25 °C?
AB(s)↽−−⇀A+(aq)+B−(aq)
Answers
The value of the solubility product constant (Ksp) for the salt AB at 25°C is 2.60 x 10⁻³.
The solubility product constant (Ksp) is the equilibrium constant for the dissolution of a sparingly soluble salt in water. It is given by the expression Ksp = [A⁺][B⁻] where [A⁺] and [B⁻] are the molar concentrations of the cations and anions in solution, respectively.
In this case, the balanced equation for the dissolution of the salt AB is: AB(s) ⇌ A⁺(aq) + B⁻(aq) We know that at 25°C, only 0.0510 mol of the salt AB is soluble in 1.00 L of water. This corresponds to a molar solubility of
s = 0.0510 mol / 1.00 L = 0.0510 M
At equilibrium, the molar concentration of A⁺ and B⁻ will also be 0.0510 M. Therefore, the value of Ksp for the salt AB at 25°C can be calculated as: Ksp = [A⁺][B⁻] = (0.0510 M) * (0.0510 M) = 2.60 x 10⁻³.
To know more about solubility product:
https://brainly.com/question/30186409
#SPJ1
Which is the middle of the three ear bones?
cochlea
stapes
incus
malleus
Answers
1. How is the law of conservation of mass shown by a balanced chemical equation?
A) The subscripts must be the same on both sides of the equation.
B) The total volumes of the substances must be the same on both sides of the equation.
C) The coefficients must be the same on both sides of the equation.
D) The number of each type of atom must be the same on both sides of the equation.
Answers
Answer:
D
Explanation:
hope this helps
Organelles called chloroplasts provide food for the plant by using the sun's energy. What
Is this process called?
A. mitochondria
B. mutation
C. photosynthesis
D. glucose

Answers
Answer:
Photosynthesis
Explanation:
Photosynthesis is a key process in plantsIn this process plants use Sunlight,water and Carbon dioxide to produce Carbohydrates .Chloroplasts take key role in it.Determine the number of carbon atoms in 1.00 kg of carbon dioxide.
____X 10__atoms(Enter your answer in scientific notation.)
Determine the number of oxygen atoms in 1.00 kg of carbon dioxide.
____X 10__atoms (Enter your answer in scientific notation.)
Calculate the mass of potassium nitrate that contains 1.00 mol of oxygen atoms.
_____g
—Pls show steps!! I’m so confused and need help—
Answers
Answer:
1.37x10²⁵atoms of carbon
2.74x10²⁵ atoms of oxygen.
33.7g of KNO₃
Explanation:
To answer this question you must use molar mass of carbon dioxide (44g/mol) and 1 mole are 6.022x10²³atoms.
1.00kg are 1000g of CO₂. Moles are:
1000g CO₂ * (1mol / 44g) = 22.73 moles of CO₂ = 22.73 moles of carbon.
In atoms:
22.73 moles C * (6.022x10²³atoms / 1mole) = 1.37x10²⁵atoms of carbon
There are 22.73 moles of CO₂ * 2 = 45.45 moles of oxygen are present in the carbon dioxide. In atoms:
45.45 moles Oxygen * (6.022x10²³atoms / 1mole) = 2.74x10²⁵ atoms of oxygen.
1 mole of Potassium nitrate, KNO₃, contains 3 moles of oxygen. 1 mol of oxygen are:
1.00 mol O * (1mol KNO₃ / 3 moles O) = 0.33 moles of KNO₃
As molar mass of KNO₃ is 101.1g/mol:
0.33 moles of KNO₃ * (101.1g / mol) = 33.7g of KNO₃
Answer:
1. 1.37 * 10^25 atoms of carbon
2. 2.74 * 10^25 atoms of oxygen
3. 33.67g of Potassium Nitrate
Explanation:
2. Firstly, we want to know the number of atoms of oxygen in 1 kg of carbon dioxide.
Firstly, we will need to know the number of moles of carbon iv oxide in 1kg of carbon iv oxide
Mathematically;
number of moles = mass/molar mass
molar mass of carbon iv oxide is 44 g/mol
mass here is 1000g (1kg is 1000g)
So the number of moles of CO2 in 1kg of CO2 will be; 1000/44 = 22.73 moles
Now 1 mole of CO2 contains 2 atoms of oxygen, thus 1 mole of CO2 contains 2 moles of oxygen
So the number of moles of oxygen in 1kg CO2 will be 2 * 22.73 = 45.46 moles
Mathematically, 1 mole contains 6.02 * 10^23 atoms
So 45.46 moles will contain =
6.02 * 10^23 * 45.46 = 2.74 * 10^25 atoms of oxygen
1. 1 mole of co2 contains 1 atom of carbon , thus, 1 mole of CO2 will
contain 1 mole of carbon
From above, 1kg of carbon iv oxide contains 22.73 moles , thus 1kg of carbon iv oxide will contain 22.73 moles of carbon too
So the number of atoms will be 22.73 * 6.02 * 10^23 = 1.37 * 10^25 atoms
3. Mass of KNO3 that contains 1 mole of oxygen atom
From the formula of the compound, we can see that 1 mole of KNO3 contains 3 atoms of oxygen which translates to 3 moles of oxygen
So 1 mole of oxygen will translate to 1/3 mole of KNO3
Mathematically;
mass = number of moles * molar mass
Molar mass of KNO3 = 39 + 14 + 3(16) = 39 + 14 + 48 = 101 g/mol
So the mass will be = 1/3 * 101 = 33.67g
What are 3 balanced chemical equations?
Answers
Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. Subscripts - Part of the chemical formulas of the reactants and products that indicate the number of atoms of the preceding element.
Examples of Balancing Chemical Equations
Example 1. C5H12 + O2 ---> CO2 + H2O. ...Example 2. Zn + HCl ---> ZnCl2 + H2 ...Example 3. Ca(OH)2 + H3PO4 ---> Ca3(PO4)2 + H2O. ...Example 4. FeCl3 + NH4OH ---> Fe(OH)3 + NH4Cl. ...Example 5. S8 + F2 ---> SF6 ...Example 6. C2H6 + O2 ---> CO2 + H2O. ...Example 7. Al2(CO3)3 + H3PO4 ---> AlPO4 + CO2 + H2O.What is the gravitational potential energy of a 1500-kg truck resting on top of a 550-m hill on earth?( earth’s gravitational pull is 9.8m/s2).
Answers
Answer:
E = 8085 kJ
Explanation:
Given that,
The mass of a truck, m = 1500 kg
Height, h = 550 m
We need to find the gravitational potential energy of the truck. It can be calculated as follows :
\(E=mgh\)
Put all the values,
\(E=1500\times 9.8\times 550\\\\E=8085000\ J\\\\or\\\\E=8085\ kJ\)
So, the gravitational potential energy is 8085 kJ.
The correct answer for the following calculation where 43 and 7 are counted numbers and 2,310 and 0.370 are measured numbers is which of the following? 43 X 2.310 7 X 0.370 a) 38.35 b) 38.4 Oc) 38 O d) 40
Answers
The actual result of the calculation is 101.920. Therefore, none of the options provided in the question matches the correct answer.
To calculate the given expression: (43 × 2.310) + (7 × 0.370), we perform the multiplication first and then add the results.
Multiplying the counted numbers:
43 × 2.310 = 99.330
Multiplying the measured numbers:
7 × 0.370 = 2.590
Now, we add the results:
99.330 + 2.590 = 101.920
The correct answer is not provided in the given options: a) 38.35, b) 38.4, c) 38, or d) 40.
The actual result of the calculation is 101.920. Therefore, none of the options provided in the question matches the correct answer.
It's important to note that when performing calculations, it is crucial to accurately follow the order of operations (multiplication before addition) and ensure precision when dealing with decimal numbers.
In this case, the correct answer is not among the options provided, and the accurate result is 101.920.
For more such questions on calculation visit:
https://brainly.com/question/28902645
#SPJ8
Question 1.4) Two bottles of the same gas are connected. One bottle has 15 particles at first and one has 2 particles. What will happen over time? Why?
Answers
We know that in gas the particles are loosely packed, that is it can move freely compared to solid and liquid. Two bottles of the same gas are connected. One bottle has 15 particles at first and one has 2 particles. Diffusion will occur. The particles will move until the volume in both the bottles will be equalized.
For gas there are three laws which are applied. Boyles law, Charles law and Avogadro's law.
Boyles law here it is the relationship between the pressure and the volume. when the volume of the gas at constant temperature varies with pressure applied on it inversely. Formula
P₁V₁=P₂V₂
Charles law is explained in the terms of temperature and volume. it states that the volume of the gas in a fixed amount is directly proportional to its temperature if and only the pressure remains constant. formula
V₁/T₁=V₂/T₂
Avogadro's law is related with the amount and the volume. it says that under the same condition the pressure and the temperature, the equal volume of different gases contains equal number of molecules.
V₁/n₁=V₂/n₂
Diffusion will occur and the particles will be same in both.
Learn more about Gas law here https://brainly.com/question/25587265
#SPJ 9
How small is an atom. Write down the calculation.
The diameter of a US penny is 19 mm. The diameter of a silver atom, by comparison, is only 2.88 *10 -10mm.
How many silver atoms could be arranged side by side in a straight line at diameter of a penny (d penny)? 10 min
d penny =19mm I # Ag = d penny /d Ag atom I # Ag=19mm /2.88 *10 -10mm = 6.6*106 atoms
d Ag atom =2.88 *10 -10mm [ 6 600 000 atoms]
# Ag atoms--->? Atoms
Answers
WE CAN SEE ATOMS!?!?! I swear I'm getting dumber everyday
but I think is like a.... a penny?
describe what xeriscaping is and what is involved in a successful xeriscaping project
Answers
Xeriscaping is a landscaping approach that focuses on conserving water by using drought-tolerant plants and efficient irrigation techniques. The goal is to create a visually appealing and sustainable garden while minimizing water usage.
Successful xeriscaping projects involve several key elements. Firstly, careful plant selection is crucial, opting for species that can thrive in arid conditions without excessive watering. Mulching is used to reduce evaporation and retain soil moisture.
Proper soil preparation, such as improving drainage and adding organic matter, promotes healthier plant growth. Efficient irrigation systems, like drip irrigation or soaker hoses, deliver water directly to plant roots, minimizing wastage.
Additionally, controlling erosion through the use of retaining walls or terracing is important. Lastly, regular maintenance, including appropriate pruning and weed control, ensures the longevity and vitality of the xeriscape garden. Overall, a successful xeriscaping project harmonizes sustainable practices with a beautiful outdoor environment.
For more such questions on Xeriscaping
https://brainly.com/question/12960529
#SPJ11
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
140 g of KCl is dissolved in 600 mL of water. What is the molarity?
Answers
Answer:
\(\boxed {\boxed {\sf molarity \approx 3 \ M \ KCl}}\)
Explanation:
Molarity is a measure of concentration in moles per liter. The formula is:
\(molarity= \frac{moles \ of \ solute}{liters \ of \ solution}\)
We are given grams of solute and liters of solution, so we must convert both before calculating molarity.
1. Convert Grams to MolesWe convert grams to moles using the molar mass. This value is found on the Periodic Table. It is the same as the atomic mass, but the units are grams per mole (g/mol) instead of atomic mass units (amu).
We have the compound KCl, so we look up the molar masses of the individual elements.
Potassium (K): 39.098 g/mol Chlorine (Cl): 35.45 g/molThe compound does not contain subscripts, so we can add the molar masses together to find the molar mass of the compound.
Potassium chloride (KCl): 39.098+ 35.45= 74.548 g/molUse the molar mass as a ratio.
\(\frac {74.548 \ g\ KCl}{1 \ mol \ KCl}\)
Multiply by 140 grams of KCl.
\(140 \ g\ KCl*\frac {74.548 \ g\ KCl}{1 \ mol \ KCl}\)
Flip the ratio so the units of grams of KCl cancel.
\(140 \ g\ KCl*\frac{1 \ mol \ KCl} {74.548 \ g\ KCl}\)
\(140 *\frac{1 \ mol \ KCl} {74.548 }\)
\(1.877984654 \ mol \ KCl\)
2. Convert Milliliters to Liters1 liter contains 1000 milliliters. Create another ratio.
\(\frac{ 1 \ L}{1000 \ mL}\)
Multiply by 600 milliliters (the value we are converting).
\(600 \ mL *\frac{ 1 \ L}{1000 \ mL}\)
The units of milliliters cancel.
\(600 \ *\frac{ 1 \ L}{1000 }\)
\(0.6 \ L\)
3. Calculate MolarityNow we know the moles of solute and the liters of solution.
1.877984654 mol KCl and 0.6 LSubstitute the values into the molarity formula.
\(molarity= \frac {1.877984654 \ mol \ KCl}{0.6 \ L}\)
\(molarity= 3.129974424 \ mol \ KCl/L\)
The original measurements of grams and milliliters have 2 and 1 significant figures. We must round our answer to the least number of sig figs: 1.
For the number we found, that is the ones place. The 1 in the tenths place tells us to leave the 3 in the ones place.
\(molarity \approx 3 \ mol \ KCl/L\)
1 mole per liter is equal to 1 molar or M. Convert the units.
\(molarity \approx 3 \ M \ KCl\)
The molarity is approximately 3 M KCl.
Given Equation (Balance it) :
C2H4O2 + NaHCO3 —> NaC2H3O2 + H2O + CO2
Word Problem:
If you have 100 mg of Acetic Acid (C2H4O2) and 10 mg of NaHCO3 (Sodium Bicarbonate), how many grams of CO2 can be produced ?
also determine the theoretical yield of the chemical reaction.
Answers
C₂H₄O₂ + NaHCO₃ —> NaC₂H₃O₂ + H₂O + CO₂ the amount of Carbon dioxide produced is 5.28 mg.
Is the reaction between acetic acid and sodium bicarbonate exothermic or endothermic?Water, CO₂ , and C₂H₃NaO₂ were produced when acetic acid and NaHCO₃ were combined. The chemistry is as follows: The reaction between vinegar and baking soda was endothermic.
Acetic acid: 2(12.01 g/mol) + 4(1.01 g/mol) + 2(16.00 g/mol)
= 60.05 g/mol
NaHCO₃ 22.99 g/mol + 1.01 g/mol + 3(16.00 g/mol)
= 84.01 g/mol
100 mg of Acetic acid is equal to 0.1 g, and 10 mg of NaHCO₃ is equal to 0.01 g.
Number of moles of Acetic acid = 0.1 g / 60.05 g/mol
= 0.00167 mol
Number of moles of NaHCO₃ = 0.01 g / 84.01 g/mol
= 0.00012 mol
Since NaHCO₃ has fewer moles, it is the limiting reactant.
Therefore, 0.00012 mol of NaHCO₃ will produce 0.00012 mol of CO₂
The mass of CO₂ produced can be calculated as follows:
Mass of CO₂ = Number of moles of CO₂ x Molar mass of CO₂
Mass of CO₂ = 0.00012 mol x 44.01 g/mol
= 0.00528 g or 5.28 mg
Therefore, the amount of CO₂ produced is 5.28 mg.
The theoretical yield of CO₂ is 0.00012 mol x 44.01 g/mol
= 0.00528 g or 5.28 mg.
This is equal to the actual yield of CO₂ produced.
To know more about Bicarbonate :
brainly.com/question/8560563
#SPJ1