a compound was analyzed and was found to contain the following percentages of the elements by mass: barium, 89.56%; oxygen, 10.44%. determine the empirical formula of the compound.
Answers
The empirical formula of the compound is BaO₅
What is empirical formula?the empirical formulate of a chemical compounds is the simple whole numbers ratio of the atoms present in the compound.
We have to determine the empirical formula of an compound that contains the following percentages by mass: O=10.44 %; % Ba=89.56%;
Empirical formula shows simplest whole number ratio of atoms in compound.
We have to find the simplest ratio of O and Ba. using ratio of percentages and atomic masses.
Atomic masses are known from periodic table: ArAr(O)=32 g/mol ArAr(Ba)= 137.3g/mol
N(O):N(Ba)= %O/Ar(O):%Ba/Ar(Ba)
N(O):N(Ba)=10.44%/32: 89.56%/137.3
N(O):N(Ba)= 0.32625:0.65229
Now we have to devide with smallest number
N(O):N(Ba)=0.32625:0.65229/0.65229
N(O):N(Ba)=5:1
The empirical formula is BaO₅
To know more about empirical formula click-
https://brainly.com/question/1603500
#SPJ4
Related Questions
The predominant intermolecular force between molecules of I₂ is _____
A) ionic bonds.
B) dipole-dipole interactions.
C) ion-dipole interactions.
D) dispersion
Answers
The correct option is C, The predominant intermolecular force between molecules of I₂ is ion-dipole interactions.
Intermolecular forces refer to the attractive or repulsive forces that occur between molecules. These forces are responsible for the physical properties of substances, including their boiling and melting points, viscosity, and surface tension.The strength of intermolecular forces varies depending on the type of molecule and the distance between them. The four main types of intermolecular forces are van der Waals forces, hydrogen bonds, dipole-dipole interactions, and ion-dipole interactions.
Van der Waals forces are the weakest and occur between all molecules, while hydrogen bonds are the strongest and occur specifically between molecules containing hydrogen and a highly electronegative atom, such as nitrogen, oxygen, or fluorine. Intermolecular forces play an important role in many chemical and physical processes, including the solubility of substances, the behavior of gases, and the properties of liquids and solids
To learn more about Intermolecular force visit here:
brainly.com/question/17111432
#SPJ4
in a hydrogen ion pump the energy is used to join small jmooelcules together to make larger ones. which factor most likely has the greatest effe ton the number of molecules
Answers
The availability of energy has the greatest impact on the number of molecules formed in a hydrogen ion pump.
In a hydrogen ion pump, the factor that most likely has the greatest effect on the number of molecules is the rate of energy supply or availability. If there is a high energy supply, more small molecules can be joined together to form larger ones. Conversely, if the energy supply is limited or insufficient, the rate of molecule formation will be lower, resulting in fewer molecules being produced. Therefore, the availability of energy plays a crucial role in determining the extent of molecule formation in a hydrogen ion pump.
You can learn more about hydrogen ion pump at
https://brainly.com/question/22591992
#SPJ11
Which situation would cause a red for an observer on the red planet?
Answers
Observations CuSO4 & NH4Cl Conventional, total ionic, net ionic
Answers
Therefore, the net ionic equation for the reaction is Copper(2+) (aq) + 2 chlorine- (aq) → Copper(II) chloride (aq).
What takes place when Copper(II) sulfate and Ammonium hydroxide interact?Ammonium sulphate and Copper hydroxide precipitate are the first products of the reaction between copper sulphate and ammonium hydroxide.
Mixing copper(II) sulphate and ammonium chloride results in the following observations:
Conventional: When copper ions (Copper2+) from Copper(II) sulfate are present, a blue solution develops. The colour of Ammonium Chloride doesn't seem to have changed at all.
Ionic total: While Ammonium Chloride dissociates into Ammonium and Chlorine- ions in solution, Copper(II) sulfate dissociates into Copper2+ and Sulfate 2- ions.
Copper(II) sulfate (aq) + 2 Ammonium Chloride (aq) → Copper(II) Chloride (aq) + 2 Ammonium (aq) + Sulfate 2- (aq)
Net Ionic: The net ionic equation shows only the species involved in the reaction. In this case, the Copper2+ and the Cl- ions combine to form Copper(II) chloride.
Copper2+ (aq) + 2 Chlorine- (aq) → Copper(II) chloride (aq)
To know more about ionic equation visit:-
https://brainly.com/question/29299745
#SPJ1
When sample X'is passed through a filter paper a
white residue, Y, remains on the paper and a clear
liquid, Z, passes through. When liquid Z is
vaporized, another white residue remains. Sample
X is best classified as

Answers
Sample X is best classified as a heterogeneous mixture
Homogenous mixture has the same uniform composition and appearance through out the reaction and it is difficult to separate the substrates present in it.
A heterogeneous mixture contains of visibly different phases or substances. Here separating the mixture becomes easy.
In the given question When sample X is passed through the filter paper we see that a white residue is remaining which is Y. This itself suggests that the the mixture is heterogeneous mixture.
As we initially got the residue it suggests that the solution is a homogenous mixture as we can now differentiate two substances
To know more about heterogenous mixtures
https://brainly.com/question/24898889
#SPJ1
Q1.what is irragation
Answers
Answer:
Irrigation is the artificial process of applying controlled amounts of water to land to assist in production of crops. Irrigation helps to grow agricultural crops, maintain landscapes, and revegetate disturbed soils in dry areas and during periods of less than average rainfall.
Answer: The watering of crop plants in regular intervals is known as irrigation. ... Weeds are unwanted plants thet grows with the crop plants. Weeding means to remove those plants. It is done manually or by machine. Using khurpi it is done manually and using seed drill it is done by machine.
Explanation:
Irrigation is the artificial application of water to the soil through various systems of tubes, pumps, and sprays. Irrigation is usually used in areas where rainfall is irregular or dry times or drought is expected.
Hope it helps
Ayúdenme por favor
Please help me
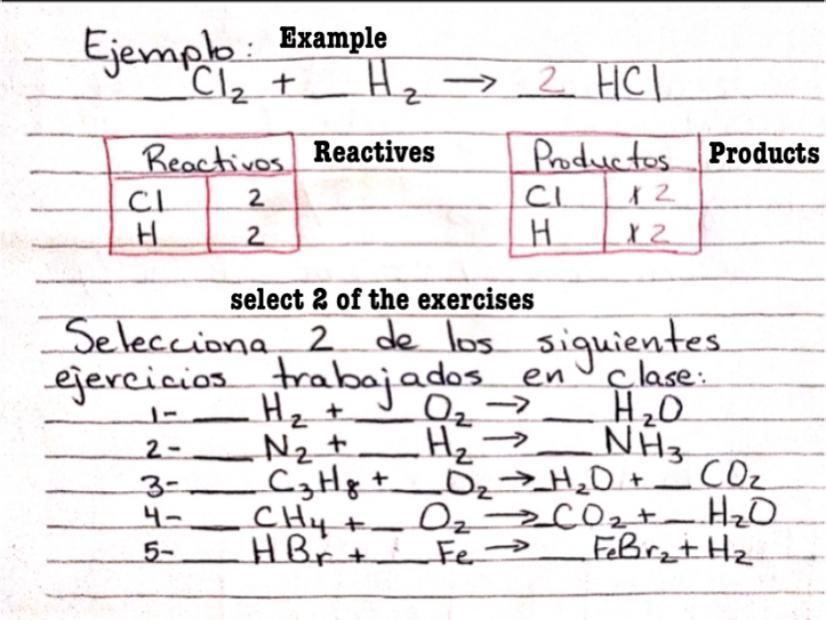
Answers
2H2+O2===>2H2O
N2+3H2==>2NH3
C3H8+5O2==>4H20+3CO2
CH4+2O2===>CO2+2H2O
2HBr +Fe==>FeBr2 +H2
I hope it helped
Define the following terms correctly.
1. Reactants-
2. Products-
3. Anion-
4. Cation-
5. Exothermic Reaction-
6. Endothermic Reaction-
Answers
1) The substances present before the reaction has occurred.
2) The substances formed after the reaction has occurred.
3) A negatively charged ion, typically non-metal, that gains electrons to become stable.
4) A positively charged ion, typically metal, that losses electrons to become stable.
5) A chemical reaction in which the reactants absorb heat energy from the surroundings to form products.
6) A chemical reaction in which the reactants release heat energy into the surroundings to form products.
Which of the following are thought to be key requirements for a world to have life?- a source of energy to fuel metabolism- a source of molecules from which to build living cells- a liquid medium
Answers
The following are thought to be key requirements for a world to have life: A source of energy to fuel metabolism, A source of molecules from which to build living cells, A liquid medium, These are the primary requirements for a world to have life. These requirements are key to the development and sustainability of life on Earth.
Every living organism requires energy to survive, and this energy comes from a variety of sources, including sunlight, food, or chemical reactions. It's necessary to have a source of energy to fuel metabolism, as it helps with the growth, development, and reproduction of an organism. A source of molecules from which to build living cells
These molecules can include things like amino acids, sugars, and lipids. A liquid medium is essential for life because it provides an environment in which chemical reactions can occur. Most chemical reactions require water to proceed, and water is also the medium in which cells operate. This is why water is considered to be the universal solvent and is an essential component for life on Earth.
Know more about reproduction here:
https://brainly.com/question/11856893
#SPJ11
what is the function of glycerin in this experiment?
Answers
In an experiment, 3.25 g of C3H8 react with 3.50 g of O2.1) Write the formula for the reactant that is the limiting reactant.
Answers
So,
The reaction that takes place here is the next one:
The first thing we're going to do is to pass the mass of each compound to moles. (We do this just dividing by the molecular weight of the compound):
Now, the last step is just to divide each amount by the coefficient of the reaction of each compound. The smaller result will be the limiting reactant.
Therefore, the limiting reactant is O2.
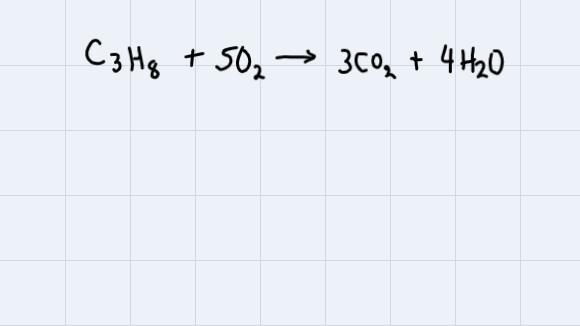


Answer the four questions to figure out the four digit code


Answers
Answer:
question 1 =c
question 2 =a
question 3 =d
question 4 =b
what makes one of your cells you ?
Answers
Part A
What question is being asked through this scientific investigation?
(I didn’t know what subject so sorry, also only answer if u have an answer.)

Answers
Answer:
How will the ingredients effect the fermentation of each bottle? What will happen to the bottles and balloons when fermentation occurs?
Explanation:
what is an orbital in chemistry
Answers
Answer:
Wave function
Explanation:
orbital, in chemistry and physics, a mathematical expression, called a wave function, that describes properties characteristic of no more than two electrons in the vicinity of an atomic nucleus or of a system of nuclei as in a molecule.
The morning temperature in a city is 41°F. If a sunny, mild day is forecast, which temperature is most likely for 2:00 p.m.?
Answers
Answer:
huh
Explanation:
An endothermic process may be spontaneous if ______. Multiple choice question. the endothermic process results in a precipitation of a crystalline solid from a liquid solution the endothermic process converts a gas reactant into a liquid or solid product the product particles have more freedom of motion than the reactant particles the product particles have less freedom of motion than the reactant particles
Answers
An endothermic process may be spontaneous if: C. the product particles have more freedom of motion than the reactant particles.
An endothermic reaction can be defined as a type of chemical reaction in which heat energy is absorbed from the environment.
Generally, heat energy is absorbed in a chemical reaction when the energy of the products is greater (more) than the energy of the reactants and this is referred to as an endothermic reaction.
Similarly, an endothermic process may be spontaneous when the product particles have more freedom of motion than the reactant particles.
Read more: https://brainly.com/question/24222328
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
Help me on this question, will give brainliest.

Answers
You know that a gas in a sealed container has a pressure of 111 kPa at 300 K. What will the
pressure be if the temperature rises to 450 K?
Answers
Indicate if each state below is either True or False: a. If a substance is oxidized, it gains electrons b. If an ion is oxidized, its oxidation number increases c. Oxidation can occur without oxygen d. Oxidation can occur without reduction
Answers
Answer:
a. False
b. True
c. False
d. True
CaCl: Will give brainliest

Answers
Answer:0.125 moles of CaCl2
Option D 0.13moles
Explanation:
Molarity= number of moles/ volume of solution
0.25M×.5 = number of moles
0.125 moles
what properties does water have that make it a very versatile fluid
Answers
Water is a very versatile fluid because it has several unique and important properties, including High heat capacity, surface tension, heat of vaporization.
Water has a high heat capacity, which means it can absorb a lot of heat energy without undergoing a large increase in temperature. This property helps to regulate the temperature of the Earth's climate and makes water an effective coolant in many industrial processes. Water has a high surface tension, which allows it to form a thin, cohesive layer on the surface of other materials. This property makes water an effective lubricant, and it helps small organisms like insects and spiders to walk on the surface of water.
Water has a high heat of vaporization, which means it requires a large amount of heat energy to change from a liquid to a gas. This property allows water to be an effective coolant, and it helps regulate the temperature of the human body through sweating.
To learn more about Water :
https://brainly.com/question/28572643
#SPJ4
NEED HELP ASAP!!! WILL MARK BRAINLIEST!!! A scuba tank can hold 18 L of air 6 atm of pressure. If the gas were to be released all underwater at 4 atm, it would take up how much volume assuming that the temperature remained constant?
Answers
\(\qquad\qquad\huge\underline{{\sf Answer}}♨\)
If temperature remains constant, then we can apply the formula :
\(\qquad \tt \dashrightarrow \:p_1v_1 = p_2v_2\)
\(\qquad \tt \dashrightarrow \:4 \times v_1 = 6 \times 18\)
\(\qquad \tt \dashrightarrow \: v_1 = 108 \div 4\)
\(\qquad \tt \dashrightarrow \:v_1 = 27\)
So, the required volume is 27 litres
25 cm of liquid 'A' and 20 cm of liquid
'B' are mixed at 25°C and the volume of
solution was measured to be 44.8 cm3
then correct reaction is
(A) A Hmix = 0, solution shows
ideal

Answers
Answer:
The correct option is;
(B) \(\Delta H_{mix} < 0\), solution shows negative deviation
Explanation:
The given parameters are;
The available volume of liquid A = 25 cm³
The available volume of liquid B = 20 cm³
The volume of the solution (mixture) = 44.8 cm³
Therefore, we have;
\(\Delta _{mix} V < 0\)
Which is one of the prerequisite for the formation of negative deviation
When a non-ideal solution shows negative deviation according to Raoult's Law, we have;
\(\Delta _{mix} H < 0\), we have more heat released due to new molecular interactions.
A yellow solid, W is soluble in water. Which method of separation can be used to obtain the solid W from an aqueous solution?
A Neutralisation
B Chromatography
С Distillation
D Crystallisation
Answers
What is the atomic mass of aluminium?
Answers
Hope this helps.
A compound that occupies a receptor but does not activate the neuron is known as a(n)?
Answers
A compound that binds to a receptor but does not activate the neuron is known as an Antagonist.
A receptor is a large protein molecule on a neuron that gets activated when a ligand binds to it such as a drug or hormone, or when electrical impulses pass through it.
An antagonist is a drug or hormone that binds to receptor, but instead of activating the receptor, it blocks or dampens the activation of the neuron. Antagonist drugs are used to interfere with the normal function or operation of a protein receptor.
Depending on the nature of the antagonist or the receptor it's bound to, the effects of antagonists may be permanent or temporary.
Learn more about antagonists here:
https://brainly.com/question/11985070
#SPJ4
what is the importance of polar covalent and hydrogen bonds in the structure of water?
Answers
Answer:
Water is a remarkable substance, and its unique properties are largely due to the presence of polar covalent bonds and hydrogen bonds in its structure. These characteristics play a crucial role in the physical and chemical properties of water, making it essential for life as we know it.
Explanation:
The polar covalent bonds in water arise from the unequal sharing of electrons between oxygen and hydrogen atoms. This results in the oxygen atom having a partial negative charge (δ-) and the hydrogen atoms having partial positive charges (δ+). These charges create polarity within the water molecule, leading to the formation of hydrogen bonds.
Hydrogen bonds occur when the partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another water molecule. These hydrogen bonds are relatively weak individually, but when present in large numbers, they contribute to the cohesion, surface tension, and high boiling point of water.
The importance of these bonds is manifold. The cohesion between water molecules due to hydrogen bonding enables water to form droplets, have a high surface tension, and flow freely, facilitating transport within organisms and in the environment. Additionally, hydrogen bonding leads to the high specific heat capacity and heat of vaporization of water, making it an effective regulator of temperature in living organisms and ensuring stable environmental conditions.
Furthermore, hydrogen bonds play a crucial role in the unique properties of water as a solvent. The polar nature of water allows it to dissolve a wide range of substances, including ionic compounds and polar molecules, facilitating various biological processes such as nutrient transport and chemical reactions in cells.
Q.) Oxidation number of P in PO4³, of S in SO4² and that of Cr in Cr₂O7² are respectively:
(a) +3, +6 and +5
(b) +5, +3 and +6
(c) +3, +6 and +6
(d) +5, +6 and +6
Answers
In a compound, the sum of the oxidation numbers of all the atoms must be equal to the overall charge on the compound. In the case of PO4³-, the overall charge is -3, so the sum of the oxidation numbers of P and O must be -3. We know that the oxidation number of O is -2, so the oxidation number of P must be +3, which is option (c).
Similarly, in the case of SO4²-, the overall charge is -2, so the sum of the oxidation numbers of S and O must be -2. We know that the oxidation number of O is -2, so the oxidation number of S must be +6, which is option (c).
Finally, in the case of Cr₂O7²-, the overall charge is -2, so the sum of the oxidation numbers of Cr and O must be -2. We know that the sum of the oxidation numbers of the O atoms is -14, so the sum of the oxidation numbers of the Cr atoms must be +12. Since there are two Cr atoms, each must have an oxidation number of +6, which is option (c).