A chemist dissolves 43.3 g Li2S (MM: 45.95 g/mol) to make 0.500 L of solution.
What is the molarity of her solution?
Answers
The molarity of her solution will be 1.88 M
What is Molarity ?Molarity is defined as the number of moles of solute dissolved in per litre of solution.
Molarity of the solution = number of moles of solute / volume of solution in litre
number of moles of solute = given weight / molecular weight
= 43.3 / 45.95
= 0.942 moles
Now, lets calculate Molarity ;
Molarity of the solution = number of moles of solute / volume of solution in litre
= 0.942 / 0.500
= 1.88 M
Hence, The molarity of her solution will be 1.88 M
Learn more about Molarity here ;
https://brainly.com/question/2817451
#SPJ1
Related Questions
Which is an example of physical weathering?
limestone in rock dissolving when acid rain flows across it
bits of rock rusting when exposed to oxygen and water
wind blowing off bits of a rock over time
oxidation of certain metals in rock
Answers
answer: bits of rock rusting when exposed to oxygen and water
Explanation: i think because when it rains the water is left and freezes and night making the rock expand or start to Rust
Which is smaller, Se, or O?
Answers
Answer:
Oxygen is much smaller then Selenium, that is because of how high the charge density is.
Answer:
If you mean electron then O(8 electrons) is smaller than Se(34 electrons).
If you mean atomic mass then O(16) is still smaller than Se(78).
If Valency then O and Se are the same(-2).
What’s the answer to this?
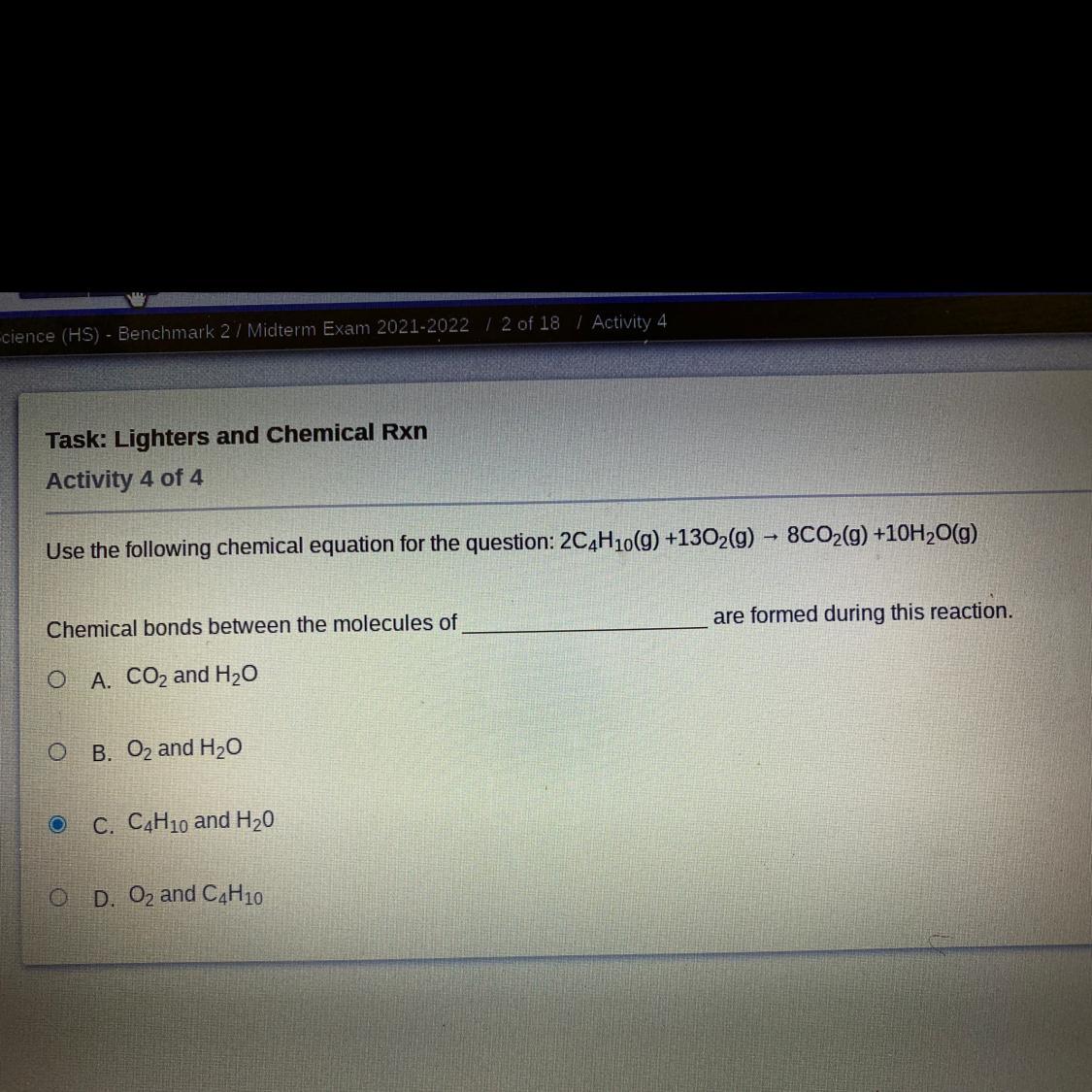
Answers
Which of the following is a Hemipteran?
Tarantula Hawk
Silverfish
June Bug
Cicada
Answers
Answer:
I think it's D, cicada.
According to Oxford, a Hemipteran is "an insect of the order Hemiptera, such as an aphid, cicada, or leafhopper."
Hope this helps! I also hope it's not wrong, but I do not think it is.
Also please let me know if this is correct.
What are the names of those?


Answers
Answer:
formula tree
Explanation:
Claim, Evidence, Reasoning : It is said that early 20th-century Italian tenor Enrico Caruso could
shatter glass with his high notes.There is no evidence to confirm this claim,
but MythBusters' Jamie Hyneman and Adam Savage tested the physics
behind this claim and found that it was possible. Write a scientific
explanation that explains what happens to cause the glass to break.
Answers
Answer: It´s question of resonance
Explanation: I think in case of crystal glass it have
certain structure. There are certain frequencies crystal can vibrate.
High sound frequencies resonate, also give energy to structure vibrations and it vibrates more strongly until structure breaks up.
Question 15
A 25.6 L sample of gas at 300 K is heated until its volume becomes 51.2 L.
Assuming pressure is constant, what will be the final temperature of the gas?
a. 500 k
b. 600k
c.300k
d.150 k
Answers
Answer:
b. 600 k
hope it help
.
.
thank you so much.
tysm
What is a simulation? Why are simulations helpful to scientists?
Answers
Answer:
A simulation is an imitation. Simulations are helpful to scientists because they allow scientists to study occurrences more closely.
Explanation:
Explanation:
A simulation is a way of imitating a process or change in the real world to predict what will happen or explain what did happen and why, these days simulations are often run on computer,scientists use simulations to answer questions see how complex system works test ideas and make predictions, hope it helps^^
btw I'm no expert, I did research^^
10. Which statement best describes evidence that a chemical reaction occurs as a cake bakes?
A The water in the cake evaporates.
B The cake rises as gas bubbles form in the baking cake.
C The cake takes the shape of the container in which it is baked.
D The water in the cake batter is used to keep the cake moist as it bakes.
Answers
Answer:
B.The cake rises as gas bubbles form in the baking cake preferably carbon dioxide
A compound can tentatively be identified by gas chromatography from its:
A. Rf value.
B. Carrier gas.
C. Partition coefficient.
D. Retention time.
Answers
Gas chromatography can be used to tentatively identify a compound from its retention time. D. Retention time.
What is chromatography? Chromatography is a method of separating out chemicals from a mixture by moving them through a material. Chromatography is a popular method for identifying and separating different chemical components in a complex mixture. The working principle of Chromatography is that the components of a mixture are separated based on their ability to adhere to a surface, i.e. the stationary phase, and their capacity to move across that surface, i.e. the mobile phase.
Gas chromatography is a powerful analytical method for the separation and quantitation of organic compounds based on their molecular characteristics. Gas chromatography is an essential method used in analytical chemistry for the qualitative and quantitative analysis of mixtures of substances. In gas chromatography, the components in a mixture are vaporized and then separated based on their distribution between a stationary liquid phase and a mobile gas phase that passes through the stationary phase. Each component is identified based on its retention time, which is the amount of time it takes for the component to travel through the column and reach the detector. Therefore, a compound can tentatively be identified by gas chromatography from its retention time. Answer: D. Retention time.
To know more about chromatography visit:
https://brainly.com/question/11960023
#SPJ11
HELP . Read the following graduated cylinder.
Use this media to help you complete the question.
22.0 mL
22 mL
23.0 mL
22.5 mL
Answers
The volume of the liquid from the graduated cylinder is 22.5 mL
The last option (22.5 mL) is the correct option
The question seems to be incomplete
The image that completes the question is shown in the attachment below.
From the image, we observe that the liquid meniscus is between the 20 mL and 25 mL marks. That means the volume will lie in this range.
NOTE: The meniscus of a liquid is the upward or downward curve seen at the top of a liquid in a container.
The type of curve shown in the image is a concave meniscus and the volume of the liquid is read from the bottom of the curve.
To read the value from the graduated cylinder, we will first determine the difference between two consecutive lines. Between the 20 mL and 25 mL mark (two thick lines), we have 5 spaces separated by thin lines.
The measure between two consecutive lines is the difference between two thick lines divided by number of spaces between them
∴ The measure between two consecutive lines = (25mL - 20mL) ÷ 5
The measure between two consecutive lines = 5mL ÷ 5 = 1 mL
Now, to read the position of the liquid,
Starting from the 20 mL mark, the liquid exceeds two more lines + half way to the next line ( that is 2mL + 0.5 mL)
∴ The position of the liquid is 20 mL + 2mL + 0.5 mL = 22.5 mL
Hence, the volume of the liquid from the graduated cylinder is 22.5 mL
Learn more here: https://brainly.com/question/19240870

Answer:
The answer is 22.5 mL
Explanation:
I hope this helps you :)
In which group of teh modern periodic table are there very reactive metals and very reactive non metals?
Answers
Metals :-
Group 1A - Alkali metals ( highly reactive metals)
Non-metals :-
Group 17 - Halogens ( highly reactive non-metals )
carbondiooxide does not support in burning but mg burs in it why.?
Answers
To keep burning, fires require oxygen. Carbon dioxide is used in certain fire extinguishers to put out flames. Explanation: Because magnesium is higher on the reactivity scale than carbon, it is more reactive and eliminates oxygen from carbon dioxide (to give carbon and magnesium oxide).
Both suspensions and colloids are heterogeneous mixtures. Define and characterize a suspension, listing similarities
and differences to a colloid. Give several examples of suspensions.
Answers
When the suspension is let to stand, the dispersed nano-particles come apart from the dispersing phase. A suspension is a uniform mixture of a single material's particles dispersed across a second stage.
What are suspensions in biology?A suspension is a homogenous mixture made up of two or more components. Most of the nanoparticles are evenly distributed throughout the liquid and thus are plain to see with the na-ked eye. Instead of dissolving in the solution, the solute's particles remain suspended there.
What occurs when there is a suspension?How a suspension works is based on the idea of force dispersion, which includes converting force into heat or eradicating the effects that force would have had. Struts, dam-pers, & springs are employed to accomplish this.
To know more about Suspensions visit:
https://brainly.com/question/17650174
#SPJ4
1. After burning the oil, there was more carbon dioxide in the glass container. Where did it come from?
2. Soybeans are plants. Where did the energy the soybean oil provides to the bus come from originally?
Please number your answer--thank you!
Answers
Answer:
1. When oil is burned, the carbon dioxide (CO2) produced comes from the carbon atoms present in the oil itself. Oil is a hydrocarbon, which means it consists of hydrogen and carbon atoms. During the combustion process, the carbon atoms combine with oxygen (O2) from the air to form carbon dioxide (CO2). So, the increased carbon dioxide in the glass container after burning the oil comes from the carbon in the oil.
2. The energy provided by soybean oil to the bus ultimately comes from the sun. Soybeans are plants that undergo a process called photosynthesis. During photosynthesis, plants use sunlight, water, and carbon dioxide from the air to produce glucose (a type of sugar) and oxygen. The glucose serves as an energy source for the plant and is stored in various forms, including oils like soybean oil. So, the energy in soybean oil can be traced back to the sun's energy captured by the plants during photosynthesis. The sun's energy is converted into chemical energy through this process, which is then transferred to the soybean oil.
Explanation:
Herbivores and carnivores are both types of consumers in a food web but they are on different trophic levels. Why are herbivores and carnivores on different trophic levels?
Herbivores have more available energy and are at a lower trophic level because they receive energy directly from producers.
Carnivores have more available energy and are at a lower trophic level because they receive energy directly from producers.
Herbivores have more available energy and are at a higher trophic level because they receive energy directly from carnivores.
Carnivores have more available energy and are at a higher trophic level because they receive energy directly from herbivores.
please help
Answers
Answer: I would say D. Carnivores have more available energy and are at a higher trophic level because they receive energy directy from herbiores.
Explanation: The grass gets its energy from the sun, the herbivore eats the grass which then gives the energy to the herbivore, then the carnivore eats the herbivore which gives the herbivore all of it's energy.
Hope This Helps!!! Have A Great Day!!!
one way to prevent carbon monoxide poisoning is to:
Answers
heating system, water heater and any other gas, oil, or coal burning appliances serviced by a qualified technician every year. Do install a battery-operated or battery back-up CO detector in your home.
when water unfreezes what happens to the volume of the sample
it increases
it decreases
it stays the same
Answers
Answer:
It eventually decreases.Explanation:
Frozen water is known to have more volume, but less density. When water slowly gets unfrozen, it'd volume decreases and its density increases. Hence, when the water gets unfrozen, it's volume decreases.
what are the 20 element
Answers
Answer:
you shoudl ask this question more clearly?
Explanation:
Answer:
The 20th Element is Calcium (Ca).
The 20 Elements are:
Hydrogen
helium
lithium
berilium
boron
carbon
nitrogen
oxygen
flourine
neon
sodium
magnesium
aluminium
silicon
phosphorus
sulphur
chlorine
argon
potassium
calcium
Choose the paramagnetic species from below.
Ar
O
Ti4+
All of the above are paramagnetic.
None of the above are paramagnetic.
Answers
The correct answer is option (c) Ti4+.
The species which are attracted to a magnetic field are known as paramagnetic species. If we talk about the given options, then we can see that there are only 3 species that are given. Out of these three, only Ti4+ is paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic. If we talk about Ti4+, then it contains 2 unpaired electrons, which makes it paramagnetic. This is the reason why the correct answer is Ti4+.In Ar, all the electrons are paired, which makes it diamagnetic. In O, there are 2 unpaired electrons, which makes it paramagnetic. How can we determine whether a species is paramagnetic or not? The species which contain unpaired electrons are paramagnetic in nature. If there are all paired electrons, then the species are diamagnetic.
Learn more about paramagnetic species at brainly.com/question/29990302
#SPJ11
Calculate the pH at 25^oC of a 0.19 M solution of potassium butanoate (KC, H,CO). Note that butanoic acid (HC, H,Co,) is a weak acid with apk, of 4.82.
Answers
The pH at 25^oC of a 0.19 M solution of potassium butanoate (KC, H,CO approximately 2.96.
Given that potassium butanoate, KC, H, CO, is a weak acid with pKa of 4.82 and a solution of 0.19 M concentration is provided, we can calculate the pH at 25°C as follows:
\(Kw = Ka × Kb\)
Kb = Kw/Ka
Where, Kw = 10^-14 (at 25°C)
Ka = 10^-pKa
We have the pKa value of potassium butanoate as 4.82.
∴ Ka = 10^-4.82
= 1.35 × 10^-5mol/L
Now, Kb = Kw/Ka
= 10^-14/1.35 × 10^-5
= 7.41 × 10^-10M
At 25°C, we can calculate the concentration of H+ ions by using the expression given below:
Ka = [H+] × [A-] / [HA]
[H+] = Ka × [HA] / [A-]
= (1.35 × 10^-5) × √0.19 / 0.19
= 1.1 × 10^-3M
Thus, pH = -log[H+]= -log(1.1 × 10^-3)≈ 2.96
Hence, the pH of 0.19 M potassium butanoate solution at 25°C is approximately 2.96.
To learn more about pH visit;
https://brainly.com/question/2288405
#SPJ11
2. (1:25) How much does the moon pull on the Earth?
A. More than the Earth's pull on the moon
B. Less than the Earth's pull on the moon
C. Equal to the Earth's pull on the moon
Answers
Answer:
a. more than the earth pull on the moon sorry if false
Which statement is true about the law of reflection?
The angle of reflected light is smaller than the incoming angle of light.
The angle of reflected light is the same as the incoming angle of light.
The angle of reflected light distorts the image to appear larger or smaller.
Answers
Answer:
the angle of reflected light distorts the image to appear larger or smaller
Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. Rate=12Δ[N2O]Δt=−12Δ[N2]Δt=Δ[O2]Δt Rate=Δ[N2O]Δt=12Δ[N2]Δt=−12Δ[O2]Δt Rate=−Δ[N2O]Δt=−12Δ[N2]Δt=12Δ[O2]Δt Rate=−12Δ[N2O]Δt=12Δ[N2]Δt=Δ[O2]Δt
Answers
The rate of reaction expressed in terms of reactants and products is; Rate=Δ[N2O]Δt=12Δ[N2]Δt=−12Δ[O2]Δt.
Rate of reactionThe term rate of reaction refers to the time taken for a reaction to take place. That is, how fast or slow a reaction progresses.
For the reaction, N2 + 1/2O2⟶N2O, we can see that the rate of reaction expressed in terms of reactants and products is; Rate=Δ[N2O]Δt=12Δ[N2]Δt=−12Δ[O2]Δt.
Learn more about rate of reaction: https://brainly.com/question/8592296
which of the following is the best description of the overall transformation inovled in the wittig reactions?
rearrangement substitution elimination addition
Answers
The overall transformation involved in the Wittig reactions can be best described as a rearrangement.
Wittig reactions are a type of organic chemical reaction that result in the formation of a new carbon-carbon double bond by converting a carbonyl compound, such as an aldehyde or ketone, into an alkene.
The process begins with the reaction of a phosphorus ylide, which is generated from a phosphonium salt, with the carbonyl compound.
The phosphorus ylide acts as a nucleophile and attacks the carbonyl carbon, leading to the formation of an intermediate called an oxaphosphetane.
This intermediate then undergoes a rearrangement, where the carbonyl oxygen is expelled, and the double bond is formed.
Therefore, the primary transformation in Wittig reactions involves the rearrangement of atoms within the molecule to produce the desired alkene product.
To know more about Wittig reactions, visit:
https://brainly.com/question/31567339
#SPJ11
why does a new flu shot need to be made each year ?
Answers
because we could be sick if don't get the shot there is a higher chance to have the flu
i hope this helps you!
6 chloroethane, c2h5cl, does not react with methanol under mild conditions. what reagent could be added to the reaction mixture to increase the rate of substitution.? a) hcl (conc.) b) naoh c) nh4oh d) agno3
Answers
The reagent that could be added to the reaction mixture to increase the rate of substitution is b NaOH.
The reaction is an example of nucleophilic substitution. The reaction is slow under mild conditions and to increase the rate of substitution, a suitable reagent needs to be added to the reaction mixture. One such reagent that could be added to increase the rate of substitution is NaOH.The addition of NaOH can increase the rate of substitution of methanol for chlorine in 6-chloroethane. It does so by deprotonating the methanol to produce the methoxide ion (CH₃O⁻) which is a stronger nucleophile than methanol. This increases the nucleophilicity of the methoxide ion which then attacks the carbon atom in the 6-chloroethane molecule. This leads to the formation of the product, methyl ethyl ether (CH₃OCH₂CH₃), as shown below:
6-chloroethane + NaOH →CH₃OCH₂CH₃ + NaCl + H₂O
NaOH acts as a base in the reaction by deprotonating methanol to generate the more reactive nucleophile, methoxide ion (CH₃O⁻).
The methoxide ion then attacks the 6-chloroethane molecule, displacing the chlorine atom and leading to the formation of the product, methyl ethyl ether (CH₃OCH₂CH₃).
Hence, the correct answer is option (b) NaOH.
To learn more about nucleophilic substitution check the link below-
https://brainly.com/question/14052597
#SPJ11
what volume of N2 is required to convert 5.0L of hydrogen gas to ammonia? assume that all gases are at the same temperature and pressure and that the reaction is complete
Answers
Answer:
Approximately \(1.7\; {\rm L}\).
Explanation:
Nitrogen \({\rm N_{2}}\, (g)\) reacts with hydrogen \({\rm H_{2}}\, (g)\) at a \(1:3\) ratio to produce ammonia \({\rm NH_3}\, (g)\):
\({\rm N_{2}}\, (g) + 3\; {\rm H_{2}}\, (g) \to 2\; {\rm NH_{3}}\, (g)\).
The ratio between the coefficient of \({\rm N_{2}}\, (g)\) and the coefficient of \({\rm H_{2}}\, (g)\) is:
\(\begin{aligned}\frac{n({\rm N_{2}})}{n({\rm H_{2}})} = \frac{1}{3}\end{aligned}\).
Under the ideal gas assumptions, the same ratio would apply to the volume of \({\rm N_{2}}\, (g)\) and \({\rm H_{2}}\, (g)\) in this reaction:
\(\begin{aligned}\frac{V({\rm N_{2}})}{V({\rm H_{2}})} = \frac{n({\rm N_{2}})}{n({\rm H_{2}})} = \frac{1}{3}\end{aligned}\).
\(\begin{aligned}V({\rm N_{2}})= \frac{1}{3}\, V({\rm H_{2}})\end{aligned}\).
Given that \(V({\rm H_{2}}) = 5.0\; {\rm L}\):
\(\begin{aligned}V({\rm N_{2}}) &= \frac{1}{3}\, V({\rm H_{2}}) \\ &= \frac{1}{3}\times 5.0\; {\rm L} \\ &\approx 1.7\; {\rm L}\end{aligned}\).
(Rounded to \(2\) significant figures.)
How does one periodic trend connect a racial trend in American Society? Why is it important to understand racial trends in American Society?
Answers
A periodic trend connect a racial trend in American Society ad it affect the distribution of power, wealth, opportunity, and create enduring social stratifications.
It is important to understand racial trends in American Society as it helps in understanding the society better.
How to illustrate the information?A trend is a shift or progression toward something new or different. Global migration is one of the most significant developments nowadays.
Today, 244 million individuals live in nations other than their native countries, which is three times the number in 1960. The world's migrant population would be the sixth largest country.
It should be noted that among the issue of racial trend is also the issue of black lives matter. This is important in order to end discrimination and also enhance the development in the country.
Learn more about American society on:
https://brainly.com/question/13323062
#SPJ1
Determine the molarity for the following solutions.
2.0 moles of potassium iodide are dissolved into 0.10 L.
20. M
10.0 grams of potassium iodide are dissolved into 100 mL.
.
0.427 moles of aluminum carbonate are dissolved into 0.855 L of water.
2340 grams of aluminum carbonate are dissolved to make 1500 mL of solution.
Calculate the asked for quantity in the following solutions.
How many grams of potassium iodide will need to be dissolved to make a 100 mL of a 2.0 M solution?
You need to make a 0.5 M solution with 25.0 grams of potassium iodide. How much water do you need to dissolve it?
How many L do you need to dissolve 10.0 grams of aluminum carbonate into a 0.3 M solution?
How many grams of aluminum carbonate do you need to dissolve to make 8.0 L of a 0.75 M solution?
Answers
The molarity, amount and volumes required are:
molarity = 20.0 MMolarity = 0.6 MMolarity = 0.5 M Molarity= 17.9 Mmass of potassium iodide = 33.2 gVolume of water needed = 0.30 L or 300 mL Volume of water needed = 0.383 L or 383 mLWhat is molarity of a solution?Molarity of a solution is defined as the number of moles of a solute dissolved in a given volume in litres of the solution.
Molarity of a solution = number of moles/volume in L Number of moles = mass/molar mass Molarity of 2.0 moles of potassium iodide are dissolved into 0.10 L.molarity = 2/0.1
molarity = 20. M
Molarityof 10 grams of potassium iodide dissolved into 100 mLmolarity mass of potassium iodide = 166 g/mol
100 mL = 0.1 L
number of moles = 10/166 = 0.06 moles
Molarity = 0.06/ 0.1
Molarity = 0.6 M
.
Molarityof 0.427 moles of aluminum carbonate dissolved into 0.855 L of water.Molarity = 0.427/0.855
Molarity = 0.50 M
Molarity of 2340 grams of aluminum carbonate dissolved to make 1500 mL of solution.1500 mL = 1.5 L
molar mass of aluminium carbonate = 87 g/mol
number of moles = 2340/87 = 26.9 moles
Molarity= 26.9/1.5
Molarity = 17.9 M
How many grams of potassium iodide will need to be dissolved to make a 100 mL of a 2.0 M solution?100 mL = 0.1 L
molarity = 2.0
number of moles = 2.0 × 0.1 = 0.2 moles
molar mass of potassium iodide = 166 g/mol
mass = number of moles × molar mass
mass = 0.2 × 166
mass of potassium iodide = 33.2 g
How much water is required to make a 0.5 M solution with 25.0 grams of potassium iodide?Number of moles of potassium iodide = 25/166 = 0.15 moles.
Volume = number of moles/molarity
Volume of water needed = 0.15/0.6
Volume of water needed = 0.30 L or 300 mL
How many L do you need to dissolve 10.0 grams of aluminum carbonate into a 0.3 M solution?Number of moles of aluminium carbonate = 10/87 = 0.115 moles.
Volume = number of moles/molarity
Volume of water needed = 0.115/0.3
Volume of water needed = 0.383 L or 383 mL
Therefore, the molarity, amount and volumes required are:
molarity = 20.0 MMolarity = 0.6 MMolarity = 0.427/0.855Molarity= 17.9 Mmass of potassium iodide = 33.2 gVolume of water needed = 0.30 L or 300 mL Volume of water needed = 0.383 L or 383 mLLearn more about molarity at: https://brainly.com/question/2927540