Answers
Answer:
273.609
Explanation:
1 mole of a substance is equal to molar mass of the substance
1 mole of substance = 106.05
2.58 moles = x
then you cross multiply to get your answer
Related Questions
How is this compound classified C4H6O4
Answers
The compound C4H6O4 can be classified as a dicarboxylic acid. In this case, the presence of four carbon atoms (C4) indicates that it is a relatively larger molecule.
The molecular formula also contains six hydrogen atoms (H6) and four oxygen atoms (O4). The presence of oxygen and carbon atoms suggests the possibility of carboxyl groups (-COOH) in the compound. Carboxyl groups are functional groups consisting of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom.
Since the compound contains four oxygen atoms, it is possible that it contains two carboxyl groups. A compound with two carboxyl groups is classified as a dicarboxylic acid. Dicarboxylic acids are organic compounds that have two carboxyl functional groups.
They are characterized by their ability to donate two protons (H+) and act as acids. Therefore, based on the molecular formula C4H6O4, the compound is classified as a dicarboxylic acid.
For more such questions on compound
https://brainly.com/question/15929599
#SPJ11
Calculate the free energy change for the reaction:
2 NO(g) + O2(g) → 2 NO2(g)
(A) - 9.3 kcal
(B) + 24.9 kcal
(C) + 9.3 kcal
(D) - 16.6 kcal
(E) + 16.6 kcal

Answers
Answer:
(D) - 16.6 kcal
Explanation:
Hello,
In this case, the Gibbs free energy for the given reaction is computed in terms of the Gibbs free energy of formation of each species involved in the chemical reaction:
\(\Delta _rG=2\Delta _fG_{NO_2}-2\Delta _fG_{NO}-\Delta _fG_{O_2}\)
Thus, it is found for nitrogen monoxide, oxygen and nitrogen dioxide the following Gibbs free energies of formation: 87.6, 0 and 51.3 kJ/mol respectively, therefore we compute:
\(\Delta _rG=2(51.3kJ/mol)-2(87.6kJ/mol)=-72.6kJ*\frac{1kcal}{4.184kJ} \\\\\Delta _rG=-17.35kcal\)
The closest result is (D) - 16.6 kcal, as such difference is noticed when different sources for thermochemical data are used, in this case, the NIST data were used.
Best regards.
The density of gold is 19.3 g/cm³. Which of the following shows the mass of a gold bar that is 720. cm³ ?
Answers
Answer:
1.39 × 10⁴ g
Explanation:
Step 1: Given data
Density of gold (ρ): 19.3 g/cm³
Volume of the gold bar (V): 720 cm³
Step 2: Calculate the mass (m) of the gold bar
The density of a substance is equal to its mass divided by its volume. This is expressed through the following expression.
ρ = m/V
m = ρ × V
m = 19.3 g/cm³ × 720 cm³
m = 1.39 × 10⁴ g
The mass of the gold bar is 1.39 × 10⁴ g.
The following reaction take place in a container where CONDITIONS ARE NOT STP! Calculate the volume nitogen dioxide that will be produced when 4,86 dm3 N2O5 decompose. 2N2O5(g) → 4NO2(g) + O2(g)
Answers
9.77 litres of NO2 are generated on average.
Calculation-The balanced equation for the breakdown of N2O5 is as follows:
\(2N_2O_5(g) -- > 4NO_2(g) + O_2(g)\)
determine how many moles of N2O5 decompose:
\(V(N_2O_5) / Vm = n(N_2O_5)(N_2O_5)\)
where V(N2O5) = 4.86 dm3 is N2O5's volume and Vm(N2O5) is N2O5's molar volume under the circumstances stated in the ideal gas law:
\((R*T)/P = Vm = V/n\)
when the gas constant R is used.
the kelvin scale of temperature, T
The pressure is P.
The ideal gas law:
\(n(N_2O_5) = V(N2O5) / Vm(N_2O_5) = 4.86 dm3 / (24.46 L/mol) = 0.1982 mol\)
the number of moles of NO2 is:
\(n(NO_2 = 4/2 * n(N_2O_5) = 0.3964 mol\)
then,
\(n(NO_2 = 4/2 * n(N_2O_5) = 0.3964 mol\)
to know more about the reaction here:
brainly.com/question/28984750
#SPJ1
If 450 grams of dextrose are dissolved in 650ml Of water with a resultant volume of 1 liter what is the percent concentration (w/v) of dextrose
Answers
Considering the definition of mass volume percentage, the percent concentration of dextrose is 45%.
Mass volume percentageMass volume percentage (% m/V) is a measure of concentration that indicates the number of grams of solute per 100 volume units of the solution. In other words, the volume percent of a component in the solution is defined as the ratio of the mass of the component to the volume of the solution, expressed as a percentage.
The mass volume percentage of a solution is determined by the following expression:
\(Mass volume percentage=\frac{mass of solute}{volume of solution}x100\)
Percent concentration in this caseIn this case, you know:
mass of solute= 450 gramsvolume of solution= 1 L= 1000 mLReplacing in the definition of mass volume percentage:
\(Mass volume percentage=\frac{450 grams}{1000 mL}x100\)
Solving:
mass volume percentage= 45%
Finally, the percent concentration of dextrose is 45%.
Learn more about mass volume percentage:
https://brainly.com/question/14471247
https://brainly.com/question/18850112
#SPJ1
Convert 150 grams of NaOH to particles of NaOH
Answers
150 grams of NaOH is approximately equal to 2.256 x 10^24 particles of NaOH.
To convert grams of NaOH to particles of NaOH, we need to use the concept of molar mass and Avogadro's number. The molar mass of NaOH is calculated by adding the atomic masses of sodium (Na), oxygen (O), and hydrogen (H) together. It can be determined as follows:
Na: 22.99 g/mol
O: 16.00 g/mol
H: 1.01 g/mol
Molar mass of NaOH = (22.99 g/mol) + (16.00 g/mol) + (1.01 g/mol) = 40.00 g/mol
Now, we can use the molar mass to convert grams of NaOH to moles. Since 1 mole contains Avogadro's number (approximately 6.022 x 10^23) particles, we can determine the number of particles as follows:
150 g NaOH * (1 mol NaOH / 40.00 g NaOH) * (6.022 x 10^23 particles / 1 mol NaOH) ≈ 2.256 x 10^24 particles
It's important to note that this calculation assumes the substance is pure NaOH and that the molar mass and Avogadro's number are accurate.
for more questions on particles
https://brainly.com/question/31213916
#SPJ11
Determine the mass in each of the following
1) 54 molecules of CO2 (MM = 44.0 g/mol)
2) 3.65 x 1024 molecules of water (MM = 18.0 g/mol)
Answers
The mass of a substance with given number of molecules can be calculated by first finding the number of moles in the substance as follows:
No. of moles = no of molecules ÷ Avogadro's number
Moles of carbon dioxide = 54 ÷ 6.02 × 10²³ = 8.97 × 10²³ molesMoles of water = 3.65 x 10²⁴ ÷ 6.02 × 10²³ = 0.61 molesMass of these substances can be calculated by multiplying the number of moles by their molar mass as follows:
Mass of carbon dioxide = 8.97 × 10²³ moles × 44 g/mol = 3.95 × 10²⁵ grams. Mass of water = 0.61 moles × 18 g/mol = 10.98 gramsLearn more about mass at: https://brainly.com/question/21042927
#SPJ1
Which of these statements is supported by the results of Thomson’s experiment? Check all of the boxes that apply
Answers
Answer:
1) Cathode rays are made up of negatively charged particles. 2)atoms contain negatively charged particles.3)Thomson's results led to the proposal of new atomic model the plum pudding model.
Explanation:
Answer:
B. Cathode rays are made up of negatively charged particles
C. Atoms contain negatively charged particles
Explanation:
Edge 2021
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
A sample of an impure ore contains 42.7% Cr2O3 by mass. What is the mass percentage of chro- mium in the ore
Answers
Answer:
29.21%
Explanation:
first of all we have to find the mass percentage of Cr in Cr2O3
molar mass of Cr2O3,
Molar Mass = 2 x Molar Mass(Cr) + 3 x Molar Mass(O)
= 2x52.0 + 3x16.0
= 104+48
= 152 g/mol
the Molar Mass of Cr2O3 = 152
we will calculate the mass of this element
mass of Cr = 2xMolarMass of (Cr)
= 104 g
Mass percentage of Cr = (104x100)/152
= 10400/152
= 68.42 %
therefore,
the fraction of Cr in ore is the fraction of Cr in Cr2O3 multi[plied by fraction of Cr2O3 in ore
42.7%
= 0.427
= 0.6842 * 0.427
= 0.292 1
when converted to %
= 29.21%
Answer: 29.21 %
what organ is very important in human body
Answers
Answer:
The brain is arguably the most important organ in the human body. It controls and coordinates actions and reactions, allows us to think and feel, and enables us to have memories and feelings—all the things that make us human.
Explanation:
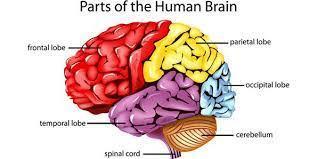
Answer:
The brain is arguably the most important organ in the human body. It controls and coordinates actions and reactions, allows us to think and feel, and enables us to have memories and feelings—all the things that make us human.
Explanation:
What volume, in liters, of 0.023 M NH4NO3 solution can be made using 25 g NH4NO3
The molar mass of NH4NO3 is 80.043 g/mol

Answers
Answer:
14 L
Explanation:
To find the volume, you need to (1) convert grams to moles (via molar mass) and then (2) calculate the volume (via molarity equation). The final answer should have 2 sig figs to match the sig figs of the given values.
(Step 1)
Molar Mass (NH₄NO₃): 80.043 g/mol
25 grams NH₄NO₃ 1 mole
------------------------------- x ------------------------- = 0.312 moles NH₄NO₃
80.043 grams
(Step 2)
Molarity = moles / volume
0.023 M = 0.312 moles / volume <----- Insert values
(0.023 M) x volume = 0.312 moles <----- Multiply both sides by volume
volume = 14 L <----- Divide both sides by 0.023
Volume, in liters, of 0.023 M NH₄NO₃ solution can be made using 25 g NH₄NO₃ are 13.47L.
What is Molarity?Molarity of a given solution is known as the total number of moles of solute per litre of the solution. A solution that is 1.00 molar (written 1.00 M) contains 1.00 mole of solute for every liter of solution.
Molarity = (No. of moles of solute ÷ Volume of solution in liters)
The unit of molarity is mol L⁻¹.
Molarity is temperature dependent because as temperature changes, volume of the solution also changes.
No. of moles of solute = Mass / Molar mass
No. of moles of solute = 0.31
Hence,
Volume of solution in liters = No. of moles of solute ÷ molarity
= 13.47 litres
Therefore, Volume, in liters, of 0.023 M NH₄NO₃ solution can be made using 25 g NH₄NO₃ are 13.47L.
Learn more about molarity, here:
https://brainly.com/question/8732513
#SPJ2
What is [H] for the solution?
x 100 M
n=
Answers
Answer:
Asumiendo
"M"
es una variable
|
Usar como
un número romano
en lugar de
Suponiendo la multiplicación
|
Uso una lista en lugar de
H x×100 M n
Figura geométrica
línea
Propiedad como función
Paridad
aun
Derivado
d/dx(H x×100 M n) = 100 H M n
Integral indefinida
integral100 H M n x dx = 50 H M n x^2 + constante
Integral definida sobre una hiperesfera de radio R
integral integral integral_(H^2 + M^2 + n^2 + x^2<R^2) 100 H M n x dH dM dn dx = 0
Integral definida sobre un hipercubo de longitud de borde 2 L
integral_(-L)^L integral_(-L)^L integral_(-L)^L integral_(-L)^L 100 H M n x dx dn dM dH = 0
Explanation:
What is the IUPAC name for the compound shown?

Answers
The IUPAC name for the compound shown is 3-ethyl-2,2-dimethylpentane.
International Union of Pure and Applied Chemistry is referred to as IUPAC. The terminology for naming organic compounds has been provided by IUPAC. The root name, prefix, and suffix are the three components that make up an IUPAC name.
There are five carbon atoms in the longest chain. Consequently, pent is the structure's root name. Choose the longest chain where the substituents are represented by the fewest numbers.
On the longest chain, three substituents are present. It consists of one ethyl group and two methyl groups. One ethyl group and two methyl groups are substituted at C-2 and C-3, respectively. Therefore, 3-ethyl-2,2-dimethyl will be the prefix. Alkane makes up the functional group. Therefore, the suffix is ane.
This ends up naming the compound as 3-ethyl-2,2-dimethylpentane.
To know more about IUPAC names, refer to the following link:
https://brainly.com/question/20488133
#SPJ9
AlCl3 + Na2SO4
Na +
Al2(SOA)
+ NaCl
what type of reaction
Answers
The Sun has been shining on this swimming pool all day. The water is much warmer than it was in the morning. Describe what is happening to the water in terms of temperature, particle speed, and kinetic energy.
Answers
Answer:
The waters' temp increased
Explanation:
The temperature of the water in the swimming pool has increased due to the heat from the Sun. As a result, the particles in the water are moving faster and have a higher kinetic energy than in the morning.
What variable must be held constant to use the combined gas law?
a. mass of gas sample
b. number of moles of gas
c. number of gas molecules
d. all of the above
e. none of the above
Answers
Answer:
The answer is (e) none of the above. The combined gas law relates the pressure, volume, and temperature of a gas, so all three variables (pressure, volume, and temperature) must be considered and at least one of them should be held constant to use the combined gas law. The mass of the gas sample, number of moles of gas, and number of gas molecules are not directly related to the combined gas law.
Consider the equations below.
A
CH (9) C(s)+2H (9) AH, = 74.6 kJ
C(s) +2012(9) ► CCI (9) AH, = -95.7 kJ
2H2(9)+2012(9) — 4HCl(9) AH2 =-184.6 kJ
CH (9)+ 4C12() → CC (9)+ 4HCI(g) AH, = -205.7 kJ
B
Complete the following based on the diagram.
D
Arrow A:
С
Arrow B:
Arrow C:
Arrow D:

Answers
74.6 kJ
exothermic
has a magnitude that is less than that of B
represents the overall enthalpy of reaction
Answer:
Arrow A:
✔ 74.6 kJ
Arrow B:
✔ exothermic
Arrow C:
✔ has a magnitude that is less than that of B
Arrow D:
✔ represents the overall enthalpy of reaction
Explanation:
edge

How are alleles and traits related?
A. Traits are segments of DNA that code for alleles, which are the
observable characteristics in an organism.
B. Alleles are the inherited characteristics that are seen through
different gene combinations.
C. Traits are characteristics inherited from parents, while alleles are
caused by the environment.
O D. Alleles are distinct versions of genes, and they code for traits,
which are distinct forms of characteristics.
Answers
Alleles and traits related as D. Alleles are distinct versions of genes, and they code for traits, which are distinct forms of characteristics.
Alleles and traits are closely related in terms of genetics and inheritance. Alleles are alternative forms of a gene that occupy the same locus on a chromosome. They represent different variations of a specific gene. Traits, on the other hand, are the observable characteristics or features of an organism that are determined by the combination of alleles.
Each individual inherits two alleles for a particular gene, one from each parent. These alleles can be the same (homozygous) or different (heterozygous). The combination of alleles determines the expression of traits in an organism. For example, in the case of eye color, the gene may have alleles for blue and brown eye color. An individual may inherit two blue alleles (homozygous), resulting in the trait of blue eyes, or they may inherit one blue and one brown allele (heterozygous), resulting in the trait of brown eyes. In summary, alleles are distinct versions of genes, and they code for the different variations of traits or characteristics that are observed in organisms. The correct answer is D. Alleles are distinct versions of genes, and they code for traits, which are distinct forms of characteristics.
for more questions on Alleles
https://brainly.com/question/16684263
#SPJ11
Identical wire loops are dipped into Liquid X and Liquid Y, so that a film of liquid forms across the loops (like the bubble solution on a child's bubble blowing wand). The width of each loop is increased slowly and the forces FX and FY needed to make the loops 5% wider are measured.
a. FX will be greater than F Y
b. FX will be less than FY
c. FX will be equal to FY
d. It's impossible to predict whether F X or FY will be greater without more information.
Answers
Answer:
a.
Explanation:
Assuming that Liquid X is considered to possess a greater viscosity as well as higher surface tension than liquid Y. Then, liquid X will tend to harbour more pressure inside the liquid.
In addition to that, the greater the surface tension, the greater the force required to expand the liquid's surface area.
This in turn makes the force required to make the loop 5% wider to be greater in FX rather than FY.
Thus, option a is the correct answer.
Seamus is conducting an experiment on electric force. He wants to get an approximate idea of how much force the charges will generate. Drag and drop the tiles to show the force of each situation in increasing order from lowest to highest (with repulsive forces being positive and attractive forces being negative).
=
One object with a charge of -4 × 10-5 C and another with a charge of 3 × 10-5 C placed 0.5
meters apart
One object with a charge of 3 x 10- C and another with a charge of -3 × 10-5 C placed 1
E
meter apart
= Two objects with a charge of 4 × 10-5 C placed 1 meter apart
= Two objects both with a charge of 3 × 10-5 C placed 0.5 meters apart
One object with a charge of 3 x 10- C and another with a charge of 4 x 10 C placed 1
E
meter apart

Answers
The highest electric force exerted by charges -4 ×10⁻⁵ C and 3 ×10⁻⁵ C placed 0.5 m apart is equal to 43.15 N.
The lowest electric force exerted by charges 3 ×10⁻⁵ C and 3 ×10⁻⁵ C placed 1 m apart is equal to 8.10 N.
What is coulomb's law?According to Coulomb’s law, the force of attraction between two charges is equal to the product of their charges and is inversely proportional to the square of the distance. This electric force applies along the line joining the two charges.
The magnitude of the electric force can be written as follows:
\(\displaystyle F = k\frac{q_1q_2}{r^2}\)
where k is constant proportionality = 8.99 × 10⁹ N.m²/C².
Given the charge on one point charge, q₁ = 4 ×10⁻⁵ C
The charge on the other point charge, q₂ = - 3 × 10⁻⁵C
The distance between these two charges, r = 0.5 m
The magnitude of electric force between the charges will be:
\(\displaystyle F = 8.99\times 10^{9}\times \frac{4\times 10^{-5}\times 3\times 10^{-5}}{(0.5)^2}\)
F = 43.15 N
Given the charge on one point charge, q₁ = 3 ×10⁻⁵ C
The charge on the other point charge, q₂ = 3 × 10⁻⁵C
The distance between these two charges, r = 1 m
The magnitude of force between the charges will be:
\(\displaystyle F = 8.99\times 10^{9}\times \frac{3\times 10^{-5}\times 3\times 10^{-5}}{(1)^2}\)
F = 8.1 N
Learn more about Coulomb's law, here:
brainly.com/question/506926
#SPJ1
A structural model of retinol is shown below. How many hydrogen atoms are
in retinol?
HC CHS
H3C
H3c
"OH
CH
A. 30
B. 23
C. 16
D. 26
Answers
Answer:
A. 30
Explanation:
Retinol is the chemical form of Vitamin A. It has a chemical formula of C20H30O.
It is processed when retinyl palmitate is broken down in the small intestine. Retinol helps in the proper regulation of eye cells hence a vital component in ensuring good eye sight.
It also helps in the neutralization of free radicals in the body and acts as an antioxidant which prevents cells of the body from ageing.
HELP ME OUT PLS!!!!!!
During the first half of the lunar cycle, the amount of light visible to Earth gets larger and larger. During this half of the cycle, the moon is said to be:
O eclipse
O rotation
O waning
O waxing

Answers
calculate the normality of a solution containing 147 g of h2s04 in 2L of solution
Answers
Explain how a rainbow is produced
Answers
A rainbow is produced through a proces that includes refraction, reflection, and dispersion of sunlight.
What more should you know about the production of rainbows?A rainbow is formed when sulinght is refracted and reflected by rain drops in the atmospher.
The sunlight is split into its component colors, which is why rainbows appear as having an array of colors. This is due to each color being bent by a different amount during refraction.
The colors of a rainbow are always in the same order, with red on the outside and violet on the inside.
Find more exercises on rainbows;
https://brainly.com/question/7965811
#SPJ1
Calculate the mass, in grams, of 532.0 atoms of cadmium, Cd (1 mol of Cd has a mass of 112.41 g).
Answers
The mass, in grams, of 532.0 atoms of cadmium is 9.93 × 10⁻²⁰ g
StoichiometryFrom the question, we are to determine the mass of the given atoms of cadmium
First, we will calculate the number of moles of cadmium present
Using the formula,
\(Number\ of\ moles = \frac{Number\ of\ atoms}{Avogadro's\ constant}\)
Then,
Number of moles of Cd present = \(\frac{532.0}{6.022 \times 10^{23} }\)
Number of moles of Cd present = 8.83427 × 10⁻²² moles
From the given information,
1 mole of Cd has a mass of 112.41 g
Therefore,
8.83427 × 10⁻²² moles will have a mass of 8.83427 × 10⁻²² × 112.41 g
8.83427 × 10⁻²² × 112.41 = 9.9306 × 10⁻²⁰ g
≅ 9.93 × 10⁻²⁰ g
Hence, the mass, in grams, of 532.0 atoms of cadmium is 9.93 × 10⁻²⁰ g
Learn more on Stoichiometry here: https://brainly.com/question/13784020
how does ease of ion pair formation depend on concentration.
Answers
The total pressure of a mixture of CO2 and O2 is 1.03 atm. If the pressure of the CO2 is 560. torr, what's the pressure of O2 in mmHg?
Answers
The pressure (in mmHg) of O₂, given that CO₂ has a pressure of 560 torr is 222.8 mmHg
We'll begin by converting 1.03 atm to torr. This can be obtained as follow:
1 atm = 760 torr
Therefore,
1.03 atm = (1.03 atm × 760 torr) / 1 atm
1.03 atm = 782.8 torr
Next, we shall determine the pressure of O₂. This can e obtained as follow:
Total pressure = 782.8 torr Pressure of CO₂ = 560 torrpressure of O₂ =?Total pressure = pressure of CO₂ + pressure of O₂
782.8 = 560 + pressure of O₂
Collect like terms
Pressure of O₂ = 782.8 - 560
Pressure of O₂ = 222.8 torr
Finally, we shall convert 222.8 torr to mmHg. This is illustrated below
Recall
760 torr = 760 mmHg
Therefore,
222.8 torr = 222.8 mmHg
Thus, we can conclude that the pressure of O₂ is 222.8 mmHg
Learn more about conversion:
https://brainly.com/question/14847010
#SPJ1
The pH at the Half-equivalence point of a weak base - strong acid tritation is:
A. Equal to pka
B. Equal to pKb
C. Less than 7.0
D. Equal to 7.0
E. Greater than 7.0
Answers
Answer:
Less than 7
Explanation:
during the titration of strong acid and weak base, the weak base is usually kept in the flask and strong acid is kept in the burette. So when we add strong acid slowly drop by drop, slowly the pH level of solution starts to decrease and at equivalence point the acid overpowers the base as a strong acid was taken over a weak base.
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M, and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant (Kc) for the given reaction is approximately 16.448. The value of Kc indicates the relative concentrations of reactants and products at equilibrium. In this case, a Kc greater than 1 suggests that the products (H2 and P4) are favored at equilibrium, indicating that the forward reaction is more favorable.
To determine the equilibrium constant (Kc) for the given reaction:
4PH3(g) ↔ 6H2(g) + P4(g)
We can write the equilibrium constant expression based on the stoichiometric coefficients:
Kc = ([H2]^6 * [P4]) / ([PH3]^4)
Substituting the given equilibrium concentrations:
[PH3] = 0.250 M
[H2] = 0.580 M
[P4] = 0.750 M
We can plug in these values into the equilibrium constant expression:
Kc = ([0.580]^6 * [0.750]) / ([0.250]^4)
Kc = (0.0860128 * 0.750) / (0.00390625)
Kc = 16.448
for more question on equilibrium
https://brainly.com/question/18849238
#SPJ8