A buffer solution is prepared that is 0.18 M NH3 and 0.27 M NH4Cl. What is the pH of this buffer? Kb for NH3 is 1.8 x 10-5
A. 4.57
B. 9.26
C. 9.43
D. 4.92
E. 9.08
Answers
Option E is Answer. The pH value of the buffer is 9.08
The pH of a solution: It is a measure of hydrogen ion concentration, which in turn is a measure of its own acidity. Pure water dissociates slightly into equal concentrations of hydrogen and hydroxyl (OH−) ions.
The pH is then calculated using the expression:
pH = - log [OH-].-------------(1)
the pH of acidic buffer =pka+log[ acid/salt]
the pH of basic buffer =pkb+log[ base/salt]
Given that Kb=1.8x10^-5
base=0.27M
salt=0.18M
first, we have to calculate the value of kb,
pkb=−log[1.8×10 ^−5]
pkb=4.74
The pH of buffer=pkb+log[base/salt]-------------(2)
We find out the value of kb and now substitute it in equation(2)
pOH=4.74+log[ 0.27/0.18]
=4.74−0.176=4.92
pH=14−4.92=9.08
To know more about the pH of solution:
https://brainly.com/question/26424076
#SPJ4
Related Questions
if 84 j of heat is added to a pure gold coin with a mass of 11 g , what is the temperature change? the specific heat capacity of gold is 0.128 j/g∘c .
Answers
Therefore, the temperature change of the pure gold coin is approximately 59.66 degrees Celsius.
To calculate the temperature change, we can use the formula:
q = m * c * ΔT
where q is the heat added, m is the mass of the gold coin, c is the specific heat capacity of gold, and ΔT is the change in temperature.
Given that q = 84 J, m = 11 g, and c = 0.128 J/g∘C, we can rearrange the formula to solve for ΔT:
ΔT = q / (m * c)
Substituting the given values:
ΔT = 84 J / (11 g * 0.128 J/g∘C)
Simplifying the expression:
ΔT = 84 J / 1.408 J/∘C
ΔT = 59.66 ∘C
Therefore, the temperature change of the pure gold coin is approximately 59.66 degrees Celsius.
To know more about temperature visit:
https://brainly.com/question/11464844
#SPJ11
The boat only moves up and down. The
wave is moving left to right. This proves
waves carry energy NOT matter.
the boat goes with the wave.
Answers
This proves waves carry energy NOT matter. the boat goes with the wave. False/
A transverse wave is a wave in which particles of the medium vibrate at proper angles, or perpendicular, to the route that the wave travels. The wake of any ship or boat consists of the waves created by means of the hull of the vessel because it acts through the water.
Waves involve the shipping of power without the shipping of remember. In conclusion, a wave may be defined as a disturbance that travels via a medium, transporting electricity from one vicinity (it's supply) to some other area without transporting count.
Learn more about transverse waves here:-transverse wave
#SPJ1
PLEASE HELP ON EASY CHEMICAL PROPERTIES

Answers
Answer:
1, just I) color.
Explanation:
Physical properties are the properties that can be observed without changing the composition of a substance, such as color, temperature, density, and boiling point.
A physical change is a change in the substance that only modifies its aggregation state, such as solidification, and boiling.
Chemical properties are the properties that need a reaction to being observed, such as combustibility, which needs a combustion reaction to being quantified.
When a chemical reaction occurs, and the composition of the substance change, it's a chemical change.
So, heating copper with carbon is a chemical reaction, and purification by electrolysis is too. Color is the only physical property.
How is iodine 131 similar to a normal atom of iodine?
Answers
Answer: Iodine 131 is a radioisotope with a very short half-life of 8.02 days, making it highly radioactive.
Explanation:
Determine the highest occupied energy level in the
following elements:
a. He
b. Be
c. Al
d. Ca
e. Sn
Answers
someone help me with this problem pls

Answers
Answer:
magnetic field
Explanation:
In an exothermic reaction is energy transferred to or from the surroundings?
Answers
Answer:
transfer energy to the surroundings
Explanation:
Exo means exit
two chemical test that can be used to differentiate propanol from propanoic acid
Answers
Propanal will form silver mirror upon heating with Tollen's reagent while propanoic acid will not respond.
What is the correct name for the compound Li2S
Answers
Answer:
Lithium sulfide
15
Which properties are characteristic of Group 2
elements at STP?
(1) good electrical conductivity and electro-
negativities less than 1.7
(2) good electrical conductivity and electro-
negativities greater than 1.7
(3) poor electrical conductivity and electro-
negativities less than 1.7
(4) poor electrical conductivity and electro-
negativities greater than 1.7
Answers
Good electrical conductivity and electronegativities less than 1.7 are the properties and characteristic of Group 2 elements at STP.
What are the properties of group 2 elements?Group 2 elements are metals so they are good conductors of heat and electricity. It has electronegativity values less than 1.7 and very reactive. They form 2+ charge in cationic form and also formed ionic bonds with other negatively charged elements.
So we can conclude that good electrical conductivity and electronegativities less than 1.7 are the properties and characteristic of Group 2 elements at STP.
Learn more about electronegativity here: https://brainly.com/question/2415812
#SPJ1
Consider the balanced equation below.
What is the mole ratio of PCl3 to PCl5?
1:1
2:1
3:5
5:3
Answers
From the balanced equation below the mole ratio of PCl3 to PCl5 is 1:1
How can the mole ration be gotten?\(PCl_{5} + PCl_{5}\) -------------------> \(PCl_{5}\)
Number of moles of \(PCl_{3}\) can be expressed as 1 mole
Number of moles of \(Cl_{2}\) can be expressed as 1 mole
Number of moles of \(PCl_{5}\) can be expressed as 1 mole
Mole ratio of \(PCl_{5}\) can be expressed as 1:1
The ratio of the mole quantities of any two compounds present in a balanced chemical reaction is known as the mole ratio. A comparison of the ratios of the molecules required to accomplish the reaction is given by the balancing chemical equation.
Learn more about mole ratio at;
https://brainly.com/question/30632038
#SPJ1
Calculate the pH of the solution:
[OH-] = 1x10^-11M
a.11.0
b.12.0
c.2.7
d.3.0
Answers
p [OH-] = -log[OH-]= -log ( 1x10^-11M) = 11
we know that
p[OH-] + p[H+] = 14
so p[H+] = 14- p[OH-] = 14- 11 = 3
pH = 3, answer
What are anthropogenic greenhouse gas emissions?
Answers
Anthropogenic greenhouse gas emissions refer to the release of greenhouse gases into the atmosphere due to human activities. These emissions mainly result from the burning of fossil fuels, deforestation, industrial processes, and agriculture. The primary greenhouse gases include carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases.
1) CO2 is the most significant anthropogenic greenhouse gas, primarily emitted from the combustion of fossil fuels such as coal, oil, and natural gas. This occurs during activities like electricity production, transportation, and manufacturing processes. Deforestation also contributes to CO2 emissions, as trees and plants absorb CO2 while they grow, and release it back into the atmosphere when they are cut down or burned.
2) Methane is another important greenhouse gas, mainly produced during the decomposition of organic matter in landfills, the digestive processes of livestock, and rice cultivation. The extraction and transport of fossil fuels, particularly natural gas, also result in methane emissions.
3) Nitrous oxide emissions primarily come from agricultural activities, such as the application of synthetic fertilizers and the management of livestock waste. Industrial processes and fossil fuel combustion also contribute to N2O emissions.
4) Fluorinated gases are synthetic chemicals used in various industrial applications, including refrigeration, air conditioning, and electronics manufacturing. Although emitted in smaller quantities, they are potent greenhouse gases with long atmospheric lifetimes.
5) Efforts to reduce anthropogenic greenhouse gas emissions include transitioning to renewable energy sources, increasing energy efficiency, and promoting sustainable land management and agriculture practices.
for more such question on Anthropogenic
https://brainly.com/question/13047496
#SPJ11
The specific heat of ethanol is 2.44 J/g ֯C. How many kJ of energy are required to heat 50.0 grams of ethanol from -20 ֯C to 68 ֯C? (heat equation)
Answers
Answer:
Heat energy required (Q) = 10.736 KJ
Explanation:
Given:
Specific heat of ethanol (C) = 2.44 J/g °C
Mass of ethanol (M) = 50 gram
Initial temperature (T1) = -20°C
Final temperature (T1) = 68°C
Find:
Heat energy required (Q) = ?
Computation:
Change in temperature (ΔT) = 68°C - (-20°C)
Change in temperature (ΔT) = 88°C
Heat energy required (Q) = mC(ΔT)
Heat energy required (Q) = (50)(2.44)(88)
Heat energy required (Q) = 10,736 J
Heat energy required (Q) = 10.736 KJ
What is the ph and the oxalate concentration of a 1. 0 m solution of oxalic acid at 25 oc? what is the concentration, in m, of other species in the equilibrium? oxalic acid is a diprotic acid with dissociation constants ka,1
Answers
Answer:
Explanation:
To calculate the pH and oxalate concentration of a 1.0 M solution of oxalic acid at 25°C, we need to use the dissociation constants (Ka) of oxalic acid. Oxalic acid is a diprotic acid, which means it can donate two protons in solution.
The dissociation constants for oxalic acid are:
Ka1 = 5.90 × 10^-2
Ka2 = 5.90 × 10^-5
To calculate the pH of a 1.0 M solution of oxalic acid, we need to first determine the concentration of hydrogen ions (H+) in solution. This can be done by considering the equilibrium reactions:
H2C2O4 ⇌ H+ + HC2O4-
HC2O4- ⇌ H+ + C2O42-
The equilibrium expressions for these reactions are:
Ka1 = [H+][HC2O4-]/[H2C2O4]
Ka2 = [H+][C2O42-]/[HC2O4-]
Since the concentration of oxalic acid is 1.0 M, the concentration of HC2O4- and C2O42- can be assumed to be negligible compared to the initial concentration of oxalic acid. Therefore, we can simplify the equilibrium expressions to:
Ka1 = [H+][HC2O4-]/1.0 M
Ka2 = [H+][C2O42-]/[HC2O4-]
Rearranging these equations and solving for [H+], we get:
[H+] = √(Ka1Ka2)/(1.0 M)
Plugging in the values for Ka1 and Ka2, we get:
[H+] = √(5.90 × 10^-2 × 5.90 × 10^-5)/(1.0 M) = 1.08 × 10^-3 M
Using the equation pH = -log[H+], we can calculate the pH of the solution:
pH = -log(1.08 × 10^-3) = 2.97
To calculate the concentration of oxalate ions (C2O42-) in solution, we can use the equilibrium expression for the second dissociation of oxalic acid:
Ka2 = [H+][C2O42-]/[HC2O4-]
We already know the concentration of hydrogen ions ([H+]) from the previous calculation, and we can assume that the concentration of HC2O4- is equal to the initial concentration of oxalic acid (1.0 M). Therefore, we can solve for [C2O42-]:
[C2O42-] = (Ka2 × [HC2O4-])/[H+]
Plugging in the values for Ka2, [HC2O4-], and [H+], we get:
[C2O42-] = (5.90 × 10^-5 × 1.0 M)/(1.08 × 10^-3 M) = 5.46 × 10^-3 M
Therefore, the concentration of oxalate ions in solution is 5.46 × 10^-3 M.
To calculate the concentrations of other species in the equilibrium, we can use the equilibrium expressions for each of the dissociation reactions and the conservation of mass balance:
[H2C2O4] = [H+] + [HC2O4-]
[HC2O4-] = [H2C2O4]/(1 + Ka1/[H+])
[C2O42-] = [HC2O4-] × Ka2/[H+]
Pl
Chemical disequilibrium is likely to be present in:_________
Answers
Chemical disequilibrium is likely to be present in any system where the forward and reverse reactions are not in balance.
This can occur in a variety of situations, such as when the reactants are not present in the correct proportions, when the reaction conditions are not ideal, or when there are external factors affecting the reaction. For example, in a chemical reaction where one product is constantly being removed from the system, the reaction may never reach equilibrium.
Similarly, in a reaction where the temperature or pressure is constantly changing, the equilibrium may shift in one direction, leading to a chemical disequilibrium. Ultimately, chemical disequilibrium occurs when a reaction is not able to maintain a stable equilibrium state. Chemical disequilibrium is likely to be present in environments where reactions are ongoing and not yet in a stable state. These situations can be found in systems experiencing changes in temperature, pressure, or concentrations of reactants and products. Examples include volcanic areas, hydrothermal vents, or chemical industries where continuous production or consumption of reactants occurs. The presence of chemical disequilibrium provides opportunities for further reactions to take place, leading to new products and potential energy releases. Understanding these environments can offer insights into various natural processes and technological applications.
To know about equilibrium:
https://brainly.com/question/30694482
#SPJ11
if a reaction solution is made up by mixing 5.0 ml of 0.0020 m fe(no3)3 ' 3.0 ml of 0.0020 m hscn, and 2.0 ml of 0.50 m hno3, the concentration of fe(no3)3 after mixing will be
Answers
After mixing 5.0 mL of 0.0020 M Fe(NO3)3, 3.0 mL of 0.0020 M HSCN, and 2.0 mL of 0.50 M HNO3, the concentration of Fe(NO3)3 will be 0.0010 M.
To find the concentration of Fe(NO3)3 after mixing, we need to calculate the total volume of the solution and the number of moles of Fe(NO3)3 present. The total volume of the solution is the sum of the volumes of the individual solutions: 5.0 mL + 3.0 mL + 2.0 mL = 10.0 mL.
First, let's calculate the moles of Fe(NO3)3:
moles of Fe(NO3)3 = volume of Fe(NO3)3 solution × concentration of Fe(NO3)3
= 5.0 mL × 0.0020 M
= 0.01 mmol (millimoles)
Since the volume is given in milliliters and the concentration is in moles per liter, we converted the volume to liters by dividing by 1000.
Now, let's calculate the final concentration of Fe(NO3)3:
concentration of Fe(NO3)3 = moles of Fe(NO3)3 / total volume of the solution
= 0.01 mmol / 10.0 mL
= 0.0010 M
Therefore, the concentration of Fe(NO3)3 after mixing will be 0.0010 M.
To know more about volume click here: brainly.com/question/32246847
#SPJ11
Can someone please help me? :(
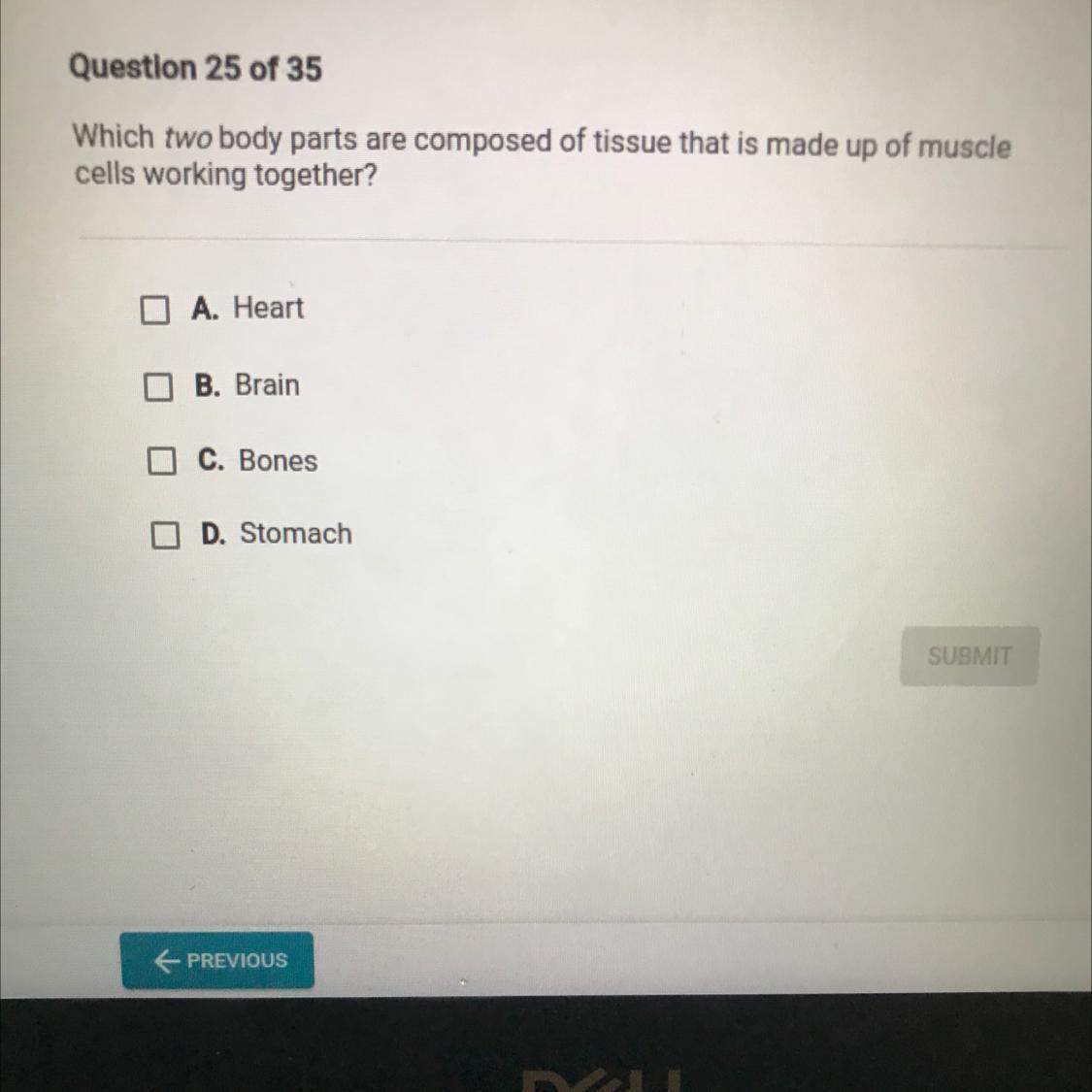
Answers
Answer:
Explanation:
I think the answer is Heart & stomach, but is this really a chemistry question? or Biology? :D
Answer:
A: heart
Explanation:
A muscle cell is also known as a myocyte when referring to either a cardiac muscle cell (cardiomyocyte), or a smooth muscle cell as these are both small cells.
how many peaks would be generated from a mass spectrometer when analyzing bromine gas?
Answers
When analyzing bromine gas using a mass spectrometer, two peaks would be generated.
A mass spectrometer is an analytical instrument used to determine the molecular weight and structural information of a substance. It operates by ionizing the sample molecules and then separating them based on their mass-to-charge ratio. In the case of bromine gas (Br2), it consists of two bromine atoms bonded together.
When bromine gas is introduced into the mass spectrometer, it undergoes ionization, typically by electron impact ionization. This process results in the formation of positively charged bromine ions (Br+). Since bromine gas contains two bromine atoms, two Br+ ions are produced.
These ions then enter the mass analyzer, where they are separated based on their mass-to-charge ratio. The mass spectrometer measures the mass of the ions, and this information is displayed as peaks in the resulting mass spectrum. Since bromine gas generates two Br+ ions, two distinct peaks would be observed in the mass spectrum when analyzing bromine gas.
Therefore, when bromine gas is analyzed using a mass spectrometer, two peaks would be generated, representing the two Br+ ions produced during ionization.
To learn more about spectrometer refer:
brainly.com/question/31518908
#SPJ11
What are the names of these molecules and what’s their molecule geometry

Answers
Answer
1. The name of the first structure is c1hloromethan and has a tTetrahedra geometry..
2. The name of the second structure is ammonium ion and has a tetrahedral geometry.
3. The name of the third structure is sulfur hexafluoride anion and has an octahedral geometry.
what is the percent yield of sulfur dioxide if the burning of 25.0 g of carbon disulfide produces 40.5 g of sulfur dioxide?
Answers
Answer:
25-54-46-36 619-73 77-88-50
How many molecules of co2 are in a 500. 0 ml container at 780 mm hg and 135°c? 8. 76 × 1021 molecules 9. 23 × 1021 molecules 5. 50 × 1021 molecules 2. 65 × 1022 molecules 2. 79 × 1022 molecules.
Answers
Step 1:
ok we have to use the formula PV=nRT
p=Pressure (must be converted to atm)= 780 mmHg
1 amt= 760 mmHg use this as a conversion factor
780 mmHg (1 atm/760 mmHg)= 1.026
V= Volume= 5.00 mL = o.5 L
n=number of moles which we have to find first
R= 0.0821
T(convert to Kelvins by adding 273.15 to the celsius temperature)= 135 C + 273.15= 408.15 k
Now plug in->
(1.026 atm)(o.5 L)= n(0.0821)(408.15 K)
(1.026 atm)(0.5 L)= n(33.509115)
(0.513)= n(33.509115)
n(number of moles)= 0.01532 mol
Now we have to convert to moles using Avagodro's number which states that 1 mol = 6.022 x 10^23 molecules or atoms
So 0.01532 mol (6.022 x 10^23 number of molesules)/ (1 mol) = 9.225704 x 10^21 = 9.226 x 10^21 colecules
Step 2
You must transfer pressure into pascals, 780 mm Hg = 103991 Pa
135*C = 408.15 k
then from the equation pV = nRt
n = pV / RT (T in Kelvins, V in M^3)
n = 103991 x 500 x 10^-6 / (8.314 x 408.15)= 0.015322 moles of N2
1 mol of everything is 6.022 x 10^23 particles, so 0.15322 moles is 0.15322 x 6.022 x 10^23 = 9.2269084 x 10^21 molecules
Explanation:
Hope this helps :)
A 150. gram sample of an unknown metal went from an initial temperature of 22.4°C to a
final temperature of 12.6°C. The sample underwent a change in thermal energy of -662 J. If the
sample is one of the metals listed in the table above, what is the identity of the metal?
Answers
Specific heat capacity if the unknown metal is -0.450 J/(g°C).
What is specific heat capacity?The measure of heat complexity needed to increase the temperature of a single unit of substance mass by one degree Celsius is known as specific heat capacity. This factor is crucial in determining how much energy is required for temperature changes in a given substance.
Equation:q = mcΔT
where q is the change in thermal energy, m is the mass of the metal, c is its specific heat capacity, and ΔT is the change in temperature.
In this case, we have:
m = 150 g
ΔT = 22.4°C - 12.6°C = 9.8°C
q = -662 J
Plugging in the values,
-662 J = (150 g) c (9.8°C)
Solving for c, we get:
c = -662 J / (150 g × 9.8°C) = -0.450 J/(g°C)
To know more about specific heat capacity, click here
https://brainly.com/question/29766819
#SPJ1
PERIODIC TABLE PLS HELP 20 POINTS

Answers
\(\bold{\huge{\blue{\underline{ Answers}}}}\)
Ans 1.) Potassium ( 1 ) , palladium (2)
Metals are those which posses lustre, hardness, malleability, ductility etc.
Ans 2.) Oxygen ( 5 ) , Argon ( 4 )
Non metals are those elements which do not possess lustre, hardness, ductility, malleability etc... they are generally brittle in nature.
Ans 3.) Boron ( 3 )
Metalloids are those substances which shows both the properties of metals and nonmetals.
Ans 4.) Boron ( 3 )
Semiconductor are those substances whose conductivity is lie between insulators and conductors.
Ans 5.) Argon(4)
Least reactive gases are those gases which generally do not reactive with any element or having very least reactivity like argon which is noble gas.
Ans 6.) Oxygen (5)
Oxygen is dull, easily breakable and good insulator but only in solid form
Ans 7.) Palladium(2)
Malleability is the property of metals that is drawn metals into thin sheets .
In an endothermic reaction, always treat heat as a
Answers
Complete the following radioactive decay problem. Please help

Answers
Answer:
230 90Th
Explanation:
A careful observation of the equation given in question shows that 234 92U is undergoing alpha decay. This means that the resulting daughter nuclei will have a decrease of 4 in the mass number and a decrease of 2 in the atomic number.
Please see attached photo for further details.
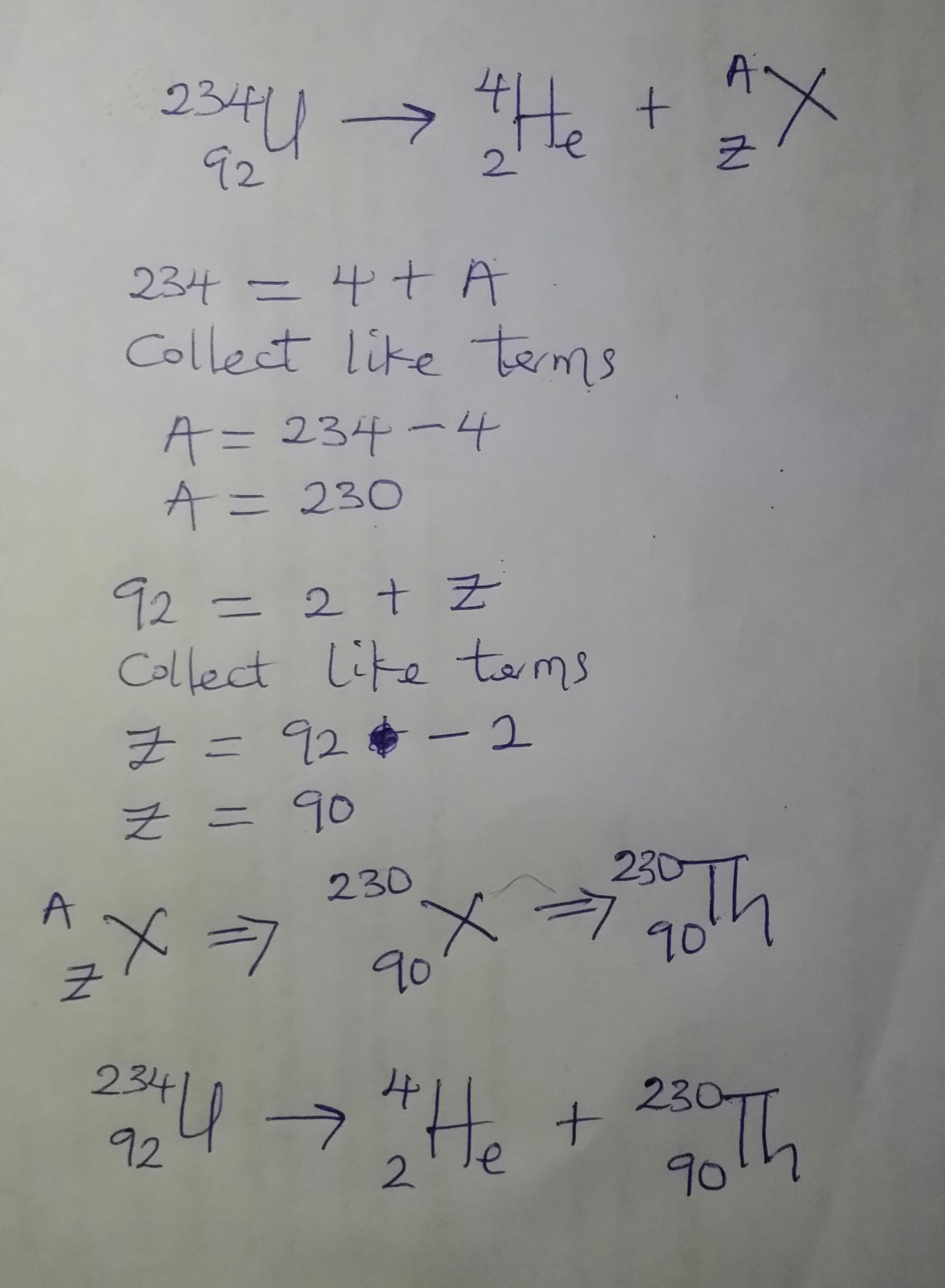
Sodium chloride is composed of molecules that are stable when dry. In water, the atoms of the molecules separate from each other. What type of chemical bond holds the dry substance together
Answers
The molecules of Sodium chloride holds a chemical bond to become stable when dry is an ionic bond.
What are chemical bonds?The chemical bonds are those which holds the atoms of molecules together and creates a temporary connection between them.
The three major types of chemical bonds are:
Ionic bondsCovalent bondsMetallic bondsWhat are ionic bonds?The ionic bonds generally occurs in between a metal and a nonmetal ions.
The atoms share electrons in between them to become stable, forms ions. Due to electric attraction between these ions forms a bond between them. That bond is called as Ionic bond.
The type of chemical bond in Sodium chloride:Sodium chloride - NaCl
Where Na(sodium) forms Na⁺ ion(metal) by sharing electrons with the chlorine atom and Cl(chlorine) forms Cl⁻ ion(non-metal).
So, due to the attraction between the anion and the cation, a bond is established between them. So, it is called Ionic bond.
Therefore, the chemical bond in Sodium chloride is an Ionic bond.
Learn more about chemical bonds here:
https://brainly.com/question/704297
#SPJ4
Which of the following statements is true?
Oxygen atoms always have a -2 oxidation state, unless it is in the elemental form O2 when it is 0.
Oxygen atoms always have a -2 oxidation state.
Oxygen atoms always have a -2 oxidations state, unless it is a peroxide such as H2O2 when it is -1, or in the element form O2 when it is 0.
Answers
The following statement is true:
Oxygen atoms always have a -2 oxidation state, unless it is in the elemental form O2 when it is 0, or in a peroxide such as H2O2 when it is -1.
This means that in most compounds containing oxygen, the oxygen atom will have an oxidation state of -2, unless it is in its elemental form (O2), in which case its oxidation state is 0. However, in peroxides such as H2O2, each oxygen atom has an oxidation state of -1.
this is from my chemistry assignment :
1) You have a tank of N2 gas. If the tank contains 11.65 L of the gas (at STP), how many molecules of N2 are in it?
can someone pls explain how I would answer this and what equation I would use ?
Answers
Answer:
Because of the gravity of the earth
Write the rate of reaction in terms of the rate of disappearance of reactant and the rate of formation of the product NO(g) +O3 _NO2(g) + O2(g)
Answers
Answer:
See Explanation
Explanation:
The rate of reaction means the same thing as the speed of a reaction. It refers to how quickly or slowly the reactants disappear or how quickly or slowly the products appear per unit time.
The equation of the reaction is; NO(g) + O3(g)→ NO2(g) + O2(g)
We can write differential equations to show the rate of disappearance of reactants or rate of appearance of products as shown below where the rate of reaction has been denoted as r;
r = -d[NO(g)]/dt = -d[O3(g)]/dt
OR
r = d[NO2(g)]/dt = d[O2(g)]
The negative signs shows that the concentration of reactants decreases with time while the positive sign shows that the concentration of products increases with time.