Answers
-6.15
ExplanationsThe formula for calculating the pH of a buffer is expressed as
\(pH=pka+log\frac{[salt]}{[acid]}^\)pka of the hydrochloric acid = -6.3
Calclate the moles of the HCl and sodium acetate
\(\begin{gathered} moles=molarity\times volume \\ moles\text{ of HCl=0.676M}\times0.275 \\ moles\text{ of HCl=0.1859moles} \end{gathered}\)Calculate the moles of sodium acetate (salt)
\(\begin{gathered} moles\text{ of sodium acetate=0.525M}\times0.500 \\ moles\text{ of sodium acetate=0.2625moles} \end{gathered}\)Substitute the given parameters into the formula
\(\begin{gathered} pH=pka+log\frac{[0.2625\text{/v}]}{[0.1859\text{/v}]} \\ pH=-6.3+log1.4121 \\ pH=-6.3+0.1498 \\ pH=-6.1502 \end{gathered}\)Hence the pH of this buffer is approximately -6.15
Related Questions
which is an example of a colloid? a mixture that settles out, a mixture that scatters light, a mixture that is separated by filtration, or a salt and water mixture?
Answers
These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Option B)
A colloid is a type of mixture in which particles are dispersed throughout a medium, creating a homogeneous appearance. Unlike solutions, where the particles are completely dissolved, and suspensions, where the particles settle out, colloids have particles that are larger than those in solutions but smaller than those in suspensions. One characteristic of colloids is that they can scatter light due to the size of the particles. This scattering of light is known as the Tyndall effect. Examples of colloids include milk, fog, and aerosol sprays. These substances have dispersed particles that are large enough to scatter light, making the beam visible. Therefore, out of the options provided, a mixture that scatters light is an example of a colloid. Therefore option B) is correct
For more question on mixture
https://brainly.com/question/24647756
#SPJ8
Note Complete Question
which is an example of a colloid?
a mixture that settles out,
b mixture that scatters light,
c mixture that is separated by filtration,
d salt and water mixture?
1. What is unsafe about Ernie's behavior?
2. Why is Lydia's behavior appropriate?
Why is it important in a laboratory setting?
Answers
Ernie's behavior is unsafe because,
In the course of playing in the laboratory, he could injure himself or damage the laboratory equipment, in this case, the microscope he is carrying.Lydia's behavior is appropriate because
As she maintains a calm and serious disposition she can get accurate results from the experiments she performs.This behavior is important because
The laboratory is a place where serious work is done. To maintain safety and achieve accuracy, Lydia's behavior is recommended.In that picture, we see Lydia well clad in her laboratory suit, and gloves. She also maintains a serious work ethic as she views the solution in the flat bottom flask.
This is the recommended attitude in the laboratory because it allows for safety and accuracy.
Ernie, on the other hand, runs around the laboratory while playing with the microscope. He could slip and have a chemical fall on him thus causing an injury.
His behavior causes distractions and might also cause mistakes for scientists observing and recording data.
Learn more here:
https://brainly.com/question/11734312
Why does water dissolve ionic compounds and solar compounds?
Answers
If a mixture of 50% liquid water and 50% ice is at zero degrees Celsius, which of the following will change if a small amount of heat is added to the mixture? (There may be more than one correct answer.) a. A small amount (not all) of the ice will melt.
b. The ratio of liquid to solid water will increase.
c. The temperature of the mixture will increase. d. The melting point for the remaining solid ice will change.
Answers
Due to the latent heat of fusion, when a modest amount of heat is added, ice will transform into water without changing the temperature. Therefore, a portion of the ice may melt (but not all of it). The right answer is A.
What exactly is fusion?The sun and stars are both powered by fusion. Fusion is the process by which two hydrogen atoms join, or fuse, to create a helium atom. Throughout the process, a portion of both the hydrogen's energy is converted into energy.
What takes place when fusion?When multiple light nuclei combine by fusion, a heavy nucleus is produced. The process results in the production of energy because as the size of the single nucleus that forms is less than the sum of the masses of the two original nuclei. Every leftover mass is transformed into energy.
To know more about fusion visit:
https://brainly.com/question/11395223
#SPJ1
The Lewis dot notation for two atoms is shown.
Mg is written with two dots on its right. O is written on the right of Mg. There are six dots around O. Two arrows point from the dots near Mg to O.
What is represented by this notation?
Mg gains two protons from O.
Mg donates two protons to O.
Mg gains two electrons from O.
Mg donates two electrons to O.
Answers
Answer:
Mg donates two electrons to O
Explanation:
Lewis dot notation uses dots and crosses to represent valence electrons on atoms.
Magnesium is a metal and would donate or lose electrons during bonding.
Oxygen is a non metal and would gain electrons during bonding.
The correct option is;
Mg donates two electrons to O
What body part is the metric system based upon?
legs
arms
fingers
feet
Answers
Answer:
arms
Explanation:
because arms can be metric system of our body
Answer:
nose and the tip of an outstretched middle finger.
Which functional group is shown below?
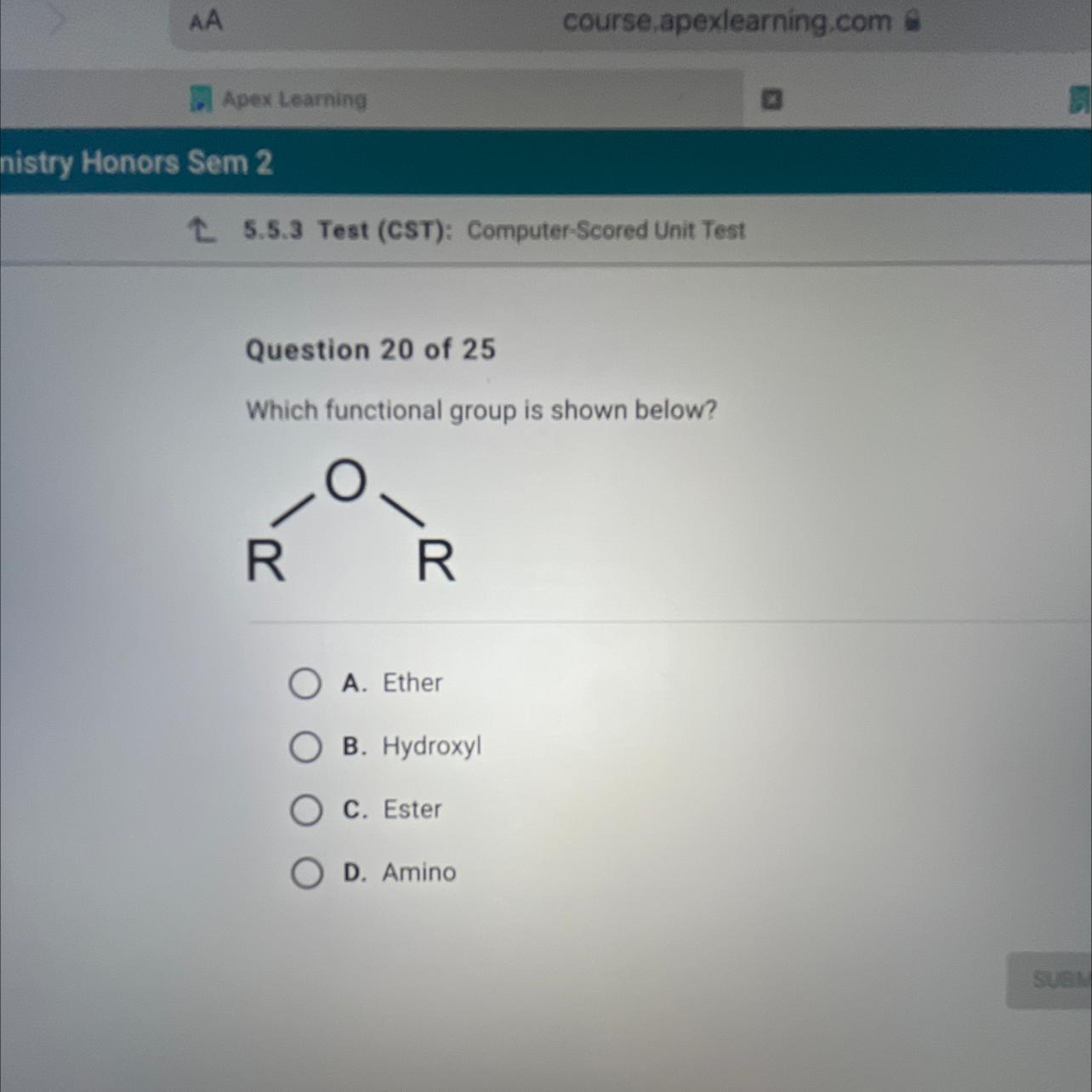
Answers
The functional group shown in the given figure in the question is ether. The correct option to this question is A.
What type of functional group is ether?Ethers. The oxygen atom that makes up the ether functional group joins two carbon atoms in a single bond. The mild reactivity of ethers makes them suitable solvents for other organic molecules.Ethers are a type of compounds in organic chemistry that have an ether group—an oxygen atom joined to two alkyl or aryl groups. They are represented by the generic formula ROR′, where R and R′ stand for the alkyl or aryl groups.The hydroxyl groups seen in alcohols are absent in ethers. Ether molecules cannot interact with one another by hydrogen bonding in the absence of the strongly polarized O-H bond. Ethers can create hydrogen bonds with other molecules (such as alcohols, amines, etc.) despite having nonbonding electron pairs on their oxygen atoms.For more information on ether kindly visit to
https://brainly.com/question/28047849
#SPJ1
MgCl,
+
K—>
balance the equation
Answers
Answer:
MgCl2 +2K= 2KCl+ Mg
Explanation:
I think MgCl2 you meant
Tools, Technology, and measurement
Answers
Answer:
Explanation:
These are daily things u use
Study the reactions for the formation of compounds from their elements

Answers
Answer
-1430 kJ
Explanation
Given information:
From 1; ΔH for the formation of 1 mol CO₂(g) = -394 kJ. For 2 mol CO₂(g), ΔH will be (2 x -394 kJ) = -788 kJ
From 2; ΔH for 1 mol H₂O(l) = -242 kJ. For 3 mol H₂O(l), ΔH will be (3 x -242 kJ) = -726 kJ
From 3, ΔH for 1 mol C₂H₆ = -84 kJ.
ΔHc for the given reaction can be calculated using the formula below:
\(ΔHc=\sum_^ΔH_f(products)-\sum_^ΔH_f(reactants)\)\(\begin{gathered} \sum_^ΔH_f(Products)=ΔH_f(2CO_2)+ΔH_f(3H_2O) \\ \\ \Rightarrow\sum_^ΔH_f(Products)=-788kJ+(-726kJ)= \\ \\ \sum_^ΔH_f(Products)=-788kJ-726kJ \\ \\ \sum_^ΔH_f(Products)=-1514kJ \end{gathered}\)For the reactants,
\(\begin{gathered} \sum_^ΔH_f(Reactants)=ΔH_f(C_2H_6)+ΔH_f(\frac{7}{2}O_2) \\ \\ \sum_^ΔH_f(Reactants)=+84+0=+84kJ \end{gathered}\)Therefore, ΔHc for the given reaction is:
ΔHc = -1514 kJ - (-84 kJ)
ΔHc = -1514kJ + 84 kJ
ΔHc = -1430 kJ

i just told the guy i like that I like him and he said he wants to get to know me better before making a decision. but now everything feels different. he's been really distant. what does that mean and how do I stop getting so attached?
Answers
Answer: you have to talk to someone who wont mind wanting to wanting to like you a lot like that.
Explanation: I wish I could be able to talk to someone who would want to get to like me like that, so its a very relatable situation.
What did Aristotle believe?
A. That matter did not exist in the physical world
B. That the scientific method should be used to test ideas
C. That all matter was composed of earth, fire, water, and air
D. That all matter was composed of tiny atoms
Answers
Answer:
C.) That all matter was composed of earth, fire, water and air
Explanation:
Just took the test
you are given experimental data below measured by a cole parmer rotary viscometer. although the fluid is unknown, you can classify the fluids as newtonian, shear thickening, and/or shear thinning. please classify each fluid.
Answers
Shear thickening fluid Fluid B, shear thinning fluid Fluid A, and Newtonian fluid Fluid C.
Describe what you mean by "fluid."Because there are more inter - particle gaps, fluids flow more fluidly and don't have a set structure. Fluids include both liquids and gases. Any fluid's molecules are always moving randomly and interacting with one another as well as any module's walls.
How many different kinds of fluid exist?Idea fluids, Real flow, Kinematic fluid, Non-Newtonian coolant, Viscoelastic secretions, and Number of initiatives Fluid are the various types of fluid. The thermodynamic characteristics of fluids are their temperatures, viscosity, pressure, as well as specific enthalpy. Physical characteristics: These characteristics, including as color and odor, aid in recognizing the fluid's physical state.
To know more about fluid visit:
https://brainly.com/question/13873557
#SPJ4
Complete question is:
You are given experimental data below measured by a Cole Parmer rotary viscometer. Although the fluid is unknown, you can classify the fluids as Newtonian, shear thickening, and/or shear thinning. Please classify each fluid. (LAB 1)
Fluid A = exponential decay
Fluid B = exponential growth
Fluid C = Linear horizontal line
Convert 550 torr into SI units.
Question 10 options:
1 atm
73,326 Pa
101,325 Pa
700 mm Hg
Answers
Answer:
73,326 Pa
Explanation:
73,326 Pa
What is the volume of an object with the mass of 7.9 grams in the density of 2.28g/ml.
Answers
Answer:
3.7mL is the volume of the object
Explanation:
To convert the mass of any object to volume we must use density that is defined as the ratio between mass of the object and the space that is occupying. For an object that weighs 7.9g and the density is 2.28g/mL, the volume is:
7.9g * (1mL / 2.28mL) =
3.7mL is the volume of the objectAmmonia is produced from the reaction of nitrogen and hydrogen according to the following balanced equation: N2(g) + 3H2(g) rightarrow 2NH3(g) What is the maximum mass of ammonia that can be produced from a mixture of 330.0 g of N2 and 420.0 g of H2? g Which element would be left partially unreacted? (enter nitrogen or hydrogen) What mass of the starting material would remain unreacted? g Look at how many grams of ammonia the N2 would produce and how many grams of ammonia the H2 would produce. Watch the mole ratios! Whichever produces the smallest amount of ammonia is the limiting reactant. HINT: After you have found which starting material was used up completely, you know that some of the other starting material is still unreacted. Subtract the reacted material (which you must calculate) from the original amount you had in the beginning, thus finding how much remains unreacted.
Answers
The maximum mass of ammonia that can be produced from a mixture of 330.0 g of N2 and 420.0 g of H2 is 400.7g. Hydrogen is left unreacted. The mass of the Hydrogen would remain unreacted is 349.3g.
What is the limiting reagent?A chemical reaction's limiting reagent is the one that will totally consume all of the reactants. The reaction cannot continue if that reactant is no longer present. It prevents the reaction from intensifying as a result.
The reactant with surplus reagent is the one that would have continued to react if the other had not been used up.
The limiting reactant is the one that is consumed first and establishes a maximum limit on the quantity of product(s) that can be produced.
Calculate the amount of each reactant present and compare it to the amount of each reactant in the balanced chemical equation to determine the limiting reactant.
1) Moles of N2=330/28=11.79
moles of H2=420/2=210
moles of NH3 formed=2*11.79=23.57moles = 400.7g
2) Hydrogen is left unreacted.
3) hydrogen left=210-11.79*3=174.64moles = 349.3g
Learn more about limiting reagent refer to ;
brainly.com/question/26905271
#SPJ4
A 0.15M solution of methylamine CH3NH2 contains an unknown concentration of methylamine chloride CH3NH3Cl. If the solution has a pH of10.20,what is the concentration of methylamine chloride in the solution? Kb for methylamine =4.6*10^-4f
Answers
The concentration of the methylamine chloride is 0.0000013 M.
What is the concentration of methylamine chloride in the solution?We know that the concentration has to do with the amount of the substance that is present. Hence, the question is essentially trying to ask us to be able to find the the concentration of methylamine chloride in the solution.
[H^+] = Antilog(-0.20) = 0.63 M
Ka = 1 * 10^-14/4.6*10^-4 = 2.22 * 10^-11
Let us first set up the ICE table as shown below;
CH3NH2(aq) + H^+ ⇄ CH3NH3^+(aq) + OH^-(aq)
I 0.15 0.63 0 0
C -x -x +x +x
E 0.15 - x 0.63 -x x x
Ka = [CH3NH3^+] [OH^-]/[CH3NH2] [H^+]
2.22 * 10^-11= x^2/[0.15 - x] [0.63 -x ]
2.22 * 10^-11(0.09 -0.15x - 0.63x + x^2) = x^2
2.22 * 10^-11(0.09 - 0.48x +x^2) = x^2
1.9 * 10^-12 - 1.1 * 10^-11x + 2.22 * 10^-11x^2 = x^2
x^2 - 2.22 * 10^-11x^2 + 1.1 * 10^-11x - 1.9 * 10^-12 = 0
x^2 + 1.1 * 10^-11x - 1.9 * 10^-12 = 0
x =0.0000013 M
Concentration of the methylamine chloride = 0.0000013 M
Learn more about concentration:https://brainly.com/question/10725862
#SPJ1
What has mass? O A. Light • B. Heat • C. Energy • D. Matter
Answers
Answer:
D. Matter
Explanation:
The definition of matter is any substance that has mass and takes up space by having volume
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
Given the reaction below, which is the being reduced?
Mg + Cl2 Right arrow. Mg2+ + 2Cl–
2CI–
CI2
Mg
Mg2+
Answers
The specie that is being reduced according to the equation is Cl2.
Now let us look at the reaction equation again;
Mg + Cl2 -----> Mg2+ + 2Cl–
Reduction occurs when there is a decrease in oxidation number and oxidation occurs when there is an increase in oxidation number.
From left to right, the oxidation number of magnesium increased from zero to +2 while the oxidation number of chlorine decreased from zero to -1.
Hence Magnesium was oxidized while chlorine was reduced.
Learn more: https://brainly.com/question/3867774
Answer:
b
Explanation:
Jai besoi daide
Completer par le mot la definition ci dessous.
1. Solute dans une solution salee
2. Solute dans un sirop
3.phenomene qui consiste a ajouter de leau dans une solution
Answers
Answer:
i dont know what you are saying
Explanation:
????????????
which formulas could represent the empirical formula and the molecular formula of a given compound?
A. CH2O, C4H6O4
B. CHO, C6H12O6
C. CH4, C5H12
D. CH2, C3H6
Answers
Answer:
d
Explanation:
d
The formulas which could represent the empirical formula and the molecular formula of a given compound are CH₂, C₃H₆ as the smallest possible is 2 which on multiplying the subscripts of empirical formula give molecular formula.
What is empirical formula?Empirical formula of a compound is defined as the simplest whole number ratio of atoms which are present in a compound.It does not make any mention of the arrangement of atoms or the number of atoms. The empirical formula gives information about the ratio of number of atoms which are present in a compound.
Molecular formula is determined from the empirical formula by dividing the molar mass of a compound by the empirical formula mass. The resultant which should be a whole number or very close to the whole number , then the subscripts are multiplied by the whole number to get the molecular formula.
Learn more about empirical formula,here:
https://brainly.com/question/14044066
#SPJ6
If 9.8 g of sulfuric acid dissolved in excess quantity moles of hydrogen ion of water, it will yield (H+) and A. 0.1, 0.2 B. 0.1, 0.3 C. 0.2, 0.4 D. 0.2,0.1 mole of sulphate ions (SO4-2)
Answers
If 9.8 g of sulfuric acid is dissolved in excess quantity moles of hydrogen ion is 0.1, 0.2. The correct option is A.
What are moles?The mole is a SI unit of measurement that is used to calculate the quantity of any substance.
Molar mass H₂SO₄ = 98 g/mol
9.8 g H₂SO₄ = 0.1 mol
H₂SO₄ produces 2 H+ ions
Therefore, [H+] = 0.2 M
Number of H+ ions = 0.2 x 6.022 x 10²³ = 1.20 x 10²³ H+ ions
H₂SO₄ dissociates in 2 steps
The first dissociation is complete because H₂SO₄ acts as a strong acid here
H₂SO₄ → H+ + HSO₄-
Thus, the 0.1 mol H₂SO₄ will produce 0.1 mol H+ and the moles of sulfate ion is 0.2. The correct option is A.
To learn more about moles, refer to the link:
https://brainly.com/question/14295820
#SPJ1
Pewter is a solidified solution of tin and lead or tin and zinc. In both cases, tin is the main component. Which metal would you classify as the solute in each type of pewter?
Answers
Which of these is not used to make models of interstellar medium?
Material from meteorites.
Minerals.
Synthetic materials.
Burnt wood ash.
Answers
Material from meteorites are not the elements used to make models of interstellar medium. The correct option is A.
What is interstellar medium?The matter and radiation that prevail in the space between star systems in a galaxy are referred to as the interstellar medium in astronomy. This matter consists of gas including ionic, atomic, and molecular, dust, and cosmic rays.
Meteorite material is not used to create models of the interstellar medium.
Thus, the correct option is A.
For more details regarding interstellar medium, visit:
https://brainly.com/question/12497791
#SPJ1
A sample of chlorine has two naturally occuring isotopes, the isotope Cl-35 makes up 75.8% of the sample, and the isotope cl-37 makes up 24.3 of the sample. Which of the following statements is true?
a. the atomic mass of chlorine will be less than 35
b. the atomic mass will be between 35 and 37
c. you can't tell what the atomic mass will be
d. the atomic mass of clorine will be more thatn 37
Answers
The statements which is true is the atomic mass will be between 35 and 37.
Isotopes can be defined as the tittles of identical rudiments that are composed of the same number of electrons and protons but differ in case of the number of neutrons. Since the number of neutrons is different in colorful isotopes of an element so, their infinitesimal millions also vary. still, the infinitesimal number remains the same as no change occurs in protons number.
Chlorine is an element in the periodic table with infinitesimal number 17 and is represented with the symbol Cl. The element nickel has colorful isotopes. One of the isotopes of chlorine is chlorine- 35 and another bone is chlorine-37 which is present in a rate of 75.8% and24.3%.
The average atomic mass of chlorine;
Average atomic mass
=(Fractional abundance of 35Cl) × (Molar Mass of 35Cl) +(Fractional abundance of 37Cl) × (Molar Mass of 37Cl)
=75.8100 × 35u + 24.3100 × 37u
=26.53u + 8.991u
=35.521 u
The average atomic mass of chlorine is 35.521 u.
Learn more about Isotopes:
https://brainly.com/question/13602441
#SPJ4
major classification of crude product
Answers
pls mark as brainlist
Explanation:
Crude is classified, based on density, as light, medium, heavy, or extra heavy. It can also be classified, based on sulfur content, into a sour and sweet category.
hexaphosphorus nonasulfide formula
Answers
Answer:
P6S9
Explanation:
Firstly, let's write the numbers in Latin
1 = mono
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
Secondly, write the symboles of the given elements:
Phosphorus is P
Sulfide is S
Finally, connect the numbers and symbols.
Rule of pronunciation: Number of first element + symbol of first element + number of second element + symbol of second element
P6S9
Please upvote.
QUESTION 1
An ion is formed when
O a. electrons are shared
b. electrons remain in place
c. neutrons are added to the nucleus
O d. electrons are gained or lost
Answers
An ion is formed electrons are gained or lost.
What is electron ?
An electron might be attached to an atom or be free. The subatomic particle known as an electron is negatively charged (not bound). In an atom, protons, neutrons, and an electron that is bound to an atom make up the three primary particle kinds. A proton, a neutron, and an electron make up an atom's nucleus.
What is ion ?
Ions are electrically charged particles that can be produced by either removing electrons from neutral atoms to produce positive ions or adding electrons to neutral atoms to produce negative ions.
Therefore, An ion is formed electrons are gained or lost.
Learn more about electron from the given link.
https://brainly.com/question/13998346
#SPJ1
Assume a healthy hearts beats exactly 70 times per minute. If individual lives to age 85 years, estimate the number of heartbeats in their lifespan using scientific notation and the correct number of sig figs.
Answers
Given :
Number of heart beat in 1 minute , n = 70 .
The individual lives for 85 years .
To Find :
The number of heartbeats in their lifespan .
Solution :
Now , number of minutes in one hour is 60 .
One day contains 24 hours .
Therefore , minutes in one whole day :
\(24\times 60 = 1440\ sec\)
Now , 1 year contains 365 days .
( Note : We are not considering any leap years )
Minutes in one year are :
\(365\times 1440=525600=5.25\times 10^5\ sec\)
So ,total minutes in 85 years is :
\(=5.25\times 10^5\times 85\\\\=4.46\times 10^7\ sec\)
Hence , this is the required solution .