Answers
The heat absorbed by the metal is 729.9 kJ.
We can calculate the heat absorbed by the copper using the formula below
⇒ Formula:
Q = cm(t₂-t₁)............... Equation 1
⇒ where:
Q = Heat absorbed by the copperc = Specific heat capacity of the copperm = Mass of the coppert₁ = Initial temperaturet₂ = Final TemperatureFrom the question,
⇒Given:
m = 6.22 kgc of copper = 385 J/kg.°Ct₁ = 20.5°Ct₂ = 325.3°C⇒Substitute these values into equation 1
Q = 6.22×385(325.3-20.5)Q = 6.22×385×304.8Q = 729904.56 JQ = 729.9 kJHence the heat absorbed by the metal is 729.9 kJ
Learn more about heat energy here: https://brainly.com/question/13439286
Related Questions
The flame from the stove the thermal energy
of the pressure cooker system, which
the
temperature.
The volume of the system
The pressure of the system
Answers
How is fission different than alpha or beta decay?
Answers
Fission is when the parent atom splits into two daughter products. Whereas atom with a mass number is lower by 4 and an atomic number is lower by 2.
Is nuclear fission alpha or beta?Fission outcomes emit beta radiation, while actinides mainly emit alpha radiation. Many of each also radiate gamma radiation.
The beta decays form an isobaric, fission-product decay chain for each mass number.
Thus, Fission is when the parent atom splits into two daughter products.
To learn more about fission click here:
https://brainly.com/question/2732120
What is the mass of a block of lead with a volume of 2,500ml?
Answers
The mass of a block of lead with a volume of 2,500ml is 28,350 grams.
How to calculate mass?The mass of a substance can be calculated using the following formula:
Density = mass ÷ volume
Density refers to the measure of the mass of matter contained by a unit volume. According to this question, a block of lead is said to have a volume of 2,500mL.
The density of lead is 11.34 g/cm³
11.34 = mass ÷ 2500
mass = 28,350grams.
Therefore, 28,350 grams is the mass of the block of lead.
Learn more about mass at: https://brainly.com/question/952755
#SPJ1
Which event takes place first in the stages before the brith of a star
Answers
Answer:
nuclear fusion
Explanation:
gravity pulls gas and dust together a protostar forms as mass increases nuclear fusion begins under high pressure
Answer options:
electric current
mass
candela
second
Kelvin
amount of a substance
volume
meter
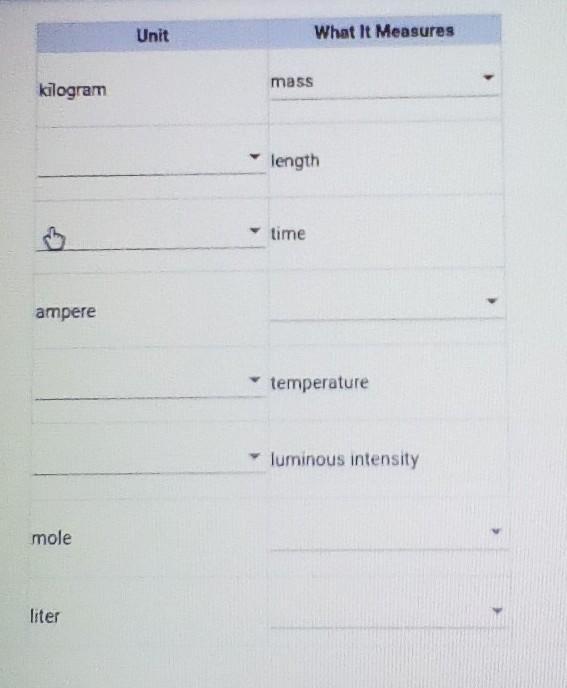
Answers
Explanation:
time ➟ second
temperature ➟ Kelvin
ampere ➟ electric current
luminous intensity ➟ candela
mole ➟ amount of a substance
liter ➟ volume
length ➟ meter
Answer:
Explanation:
Kilogramm → mass
Ampere → electric current
Mole → amount of a substance
Liter → volume
the earth is 24,901 miles. how many hours would it take to travel around the earth if traveling 100 meters per second?
Answers
Answer:
24,901 x 1609.34 = 40074275
40074275/100 equals
400742.75 s
divided by 3600
111.31
Explanation:
1)
Radioactive materials have unstable
A)
nuclei
B)
electrons.
Delectron clouds.
D)
area of atom outside of nucleus.
Answers
Because of extra protons and neutrons
Not divisible
O atom
O compound
O mixture
O solution
Answers
Answer:
mixture or atom
Explanation:
I HOPE IT WILL HELP YOU
Explanation:
and ,answer is mixture.
which of the indicated protons in the following compound would appear most upfield in the 1h nmr spectrum?
Answers
P1 proton in the compound appear most upfield in the 1H NMR spectrum.
What is the main principle of NMR?
The foundation of NMR is the idea that all nuclei are electrically charged and that many of them have spins. Energy can go from the base energy to a higher energy level if an external magnetic field is provided.
The protons are most shielded ones among all the four types of protons as they experience +I effect from adjacent CH2 groups and are also situated far away from carbonyl group. therefore the -I effect is very less compared to the protons 2, 3 and 4. all these factors makes the proton 1 the most shielded and hence they will have very low delta value that is upfield.
Therefore, P1 proton in the compound appear most upfield in the 1H NMR spectrum.
To learn more about NMR spectroscopy from the given link.
https://brainly.com/question/21024524
#SPJ4
A study of the Earth by The National Aeronautics and Space Administration (NASA) determined there were 8.80×10¹¹ tons of
carbon stored in living plants on the Earth's surface, at the time of the study. Calculate the mass of carbon stored in these plants
in grams.
Answers
The mass of carbon stored in the plants in grams is 7.98×10¹⁴ Kg
Data obtained from the questionFrom the question given above, the following data were obtained:
Carbon (in ton) = 8.80×10¹¹ tons Carbon (in Kg) =?How to convert 8.80×10¹¹ tons to kilograms (Kg)We can convert 8.80×10¹¹ tons to kilograms as illustrated below:
1 ton = 907.19 Kg
Therefore,
8.80×10¹¹ tons = (8.80×10¹¹ tons × 907.19 Kg) / 1 ton
8.80×10¹¹ tons = 7.98×10¹⁴ Kg
Thus, 8.80×10¹¹ tons is equivalent to 7.98×10¹⁴ Kg
Therefore, we can say that the mass of the carbon in the plants is 7.98×10¹⁴ Kg
Learn more about conversion:
https://brainly.com/question/12974609
#SPJ1
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
Continual eruptions occur along mid-ocean ridges, forming new sea-floor rock. The
rocks closest to a mid-ocean ridge are the youngest | oldest. As you move away from
a mid-ocean ridge, the rocks get younger | older. This implies that crust is being
consumed | produced at mid-ocean ridges
Answers
The characteristics of the ocean ridges allow finding the results to complete the statements are:
The rocks near the ridge are YOUNGER. While you walk away the rocks are OLDER. This implies that the cortex is PRODUCING in the ridges.
The oceanic ridges are mountain ranges or chains of volcanic mountains at the bottom of the ocean, in these the magma that rises cools and forms young rocks, in they are pushed by the other rocks that emerge, in general they are located on the edges of the tectonic plates .
Therefore, the oceanic ridges are the point where the earth's crust is separating, therefore it has the youngest rocks and in the furthest points it has the oldest.
Let's find the correct words for each phrase to be correct:
The rocks near the ridge are YOUNGER. You walk away the rocks are OLDER. This implies that the cortex is PRODUCING in the ridges.
As a consequence of the characteristics of the ocean ridges, we can find the results to complete the statements are:
The rocks near the ridge are YOUNGER. While you walk away the rocks are OLDER. This implies that the cortex is PRODUCING in the ridges.
Learn more here: brainly.com/question/3404002

What are properties of a base
Answers

Answer:
base taste bitterbase feel so spy slippery to touchbase turn wet red litmus paper blueTrees cover what percentage of the Smoky Mountains National Park.
A. 95 percent
B. 87 Percent
C.65 percent
D.34 percent
Answers
Answer:
C. 65 percent
What is the change in enthalpy when 90.6 g
of steam at 100◦C is converted to liquid water
at the same temperature and pressure? The
heat of vaporization of water is 40.7 kJ/mole.
Answers
The change in enthalpy when 90.6 g of steam at 100◦C is converted to liquid water at the same temperature and pressure is 204.7 KJ
How do i determine the change in enthalpy?First, we shall obtain the number of mole water converted to steam. details below:
Mass of water = 90.6 grams Molar mass of water = 18 g/mol Mole of water =?Mole = mass / molar mass
Mole of water = 90.6 / 18
Mole of water = 5.03 moles
Finally, we shall determine the change in enthalpy. Details below:
Mole of water (n) = 5.03 molesHeat of vaporization (ΔHv) = 40.7 KJ/molChange in enthalpy (ΔH) =?ΔH = n × ΔHv
ΔH = 5.03 × 40.7
ΔH = 204.7 KJ
Thus, we can conclude that the change in enthalpy is 204.7 KJ
Learn more about enthalpy change:
https://brainly.com/question/24170335
#SPJ1
What is the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C? A) 4.48 x 10¹¹ atm B) 2.24 x 10⁰ atm C) 1.12 x 10³ atm D) 2.24 x 10³ atm
Answers
The pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C is 2.24 × 10⁰ atm.
How to calculate pressure?The pressure of a substance can be calculated using the following formula;
PV = nRT
P = pressureV = volumen = no of molesR = gas law constantT = temperatureAccording to this question, the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C can be calculated as follows:
P × 2 = 0.1 × 0.0821 × 546
2P = 4.48266
P = 2.24 × 10⁰ atm
Learn more about pressure at: https://brainly.com/question/31525061
#SPJ1
You are working in a lab when radiation alarms go off. You are able to hide inside a steel cabinet, whose sides are about 1.5 inches thick, until the alarm goes off. Preliminary reports show that the radiation was weakly ionizing and negatively charged. Were you safe in the cabinet?
A. Yes, it was beta radiation and the steel was enough to block it.
B. Yes, it was alpha radiation and not harmful.
C. No, it was beta radiation and very dangerous.
D. No, it was gamma radiation and can only be blocked by a thick wall of lead.
Answers
Answer
A
Explanation:
Beta radiation is only very dangerous when it is ingested or if there is nothing in the way. The steel should protect you.
Yes, it was beta radiation and the steel was enough to block it. Hence, option A is correct.
What is beta radiation?Beta radiation is a current of electrons released at a rate exceeding the speed of light, with kinetic energy between 0.2 MeV and 3.2 MeV. Due to their lower mass which is approximately 5.5×\(10^{-4}\) amu (9.130×\(10^(-24)\), interactions between β-particles and the atoms of pass-through materials are much less frequent than the interactions between 5-007-particles: less than 200 ion pairs are typically formed in each centimetre of the air passage.
When we are working in a lab when radiation alarms go off and we are able to hide inside a steel cabinet, whose sides are about 1.5 inches thick, until the alarm goes off.
Preliminary reports show that the radiation was weakly ionizing and negatively charged. Yes, it was beta radiation and the steel was enough to block it.
Hence, option A is correct.
Learn more about beta radiation here:
https://brainly.com/question/16645044
#SPJ5
Write a net ionic equation for the reaction that occurs when aqueous solutions of hydrofluoric acid and barium hydroxide are combined. Include solubility states
Answers
Answer: The net ionic equation is \(2H^+(aq)+2F^-(aq)+Ba^{2+}(aq)+2OH^-(aq)\rightarrow BaF_2(s)+2H_2O(l)\)
Explanation:
The net ionic equation of any reaction does not include any spectator ions.
Spectator ions are defined as ions that do not get involved in a chemical equation. They are found on both the sides of the chemical reaction when it is present in ionic form.
The chemical equation for the reaction of hydrofluoric acid and barium hydroxide follows:
\(2HF(aq)+Ba(OH)_2(aq)\rightarrow BaF_2(s)+2H_2O(l)\)
The ionic form of the above equation follows:
\(2H^+(aq)+2F^-(aq)+Ba^{2+}(aq)+2OH^-(aq)\rightarrow BaF_2(s)+2H_2O(l)\)
There are no spectator ions in the ionic form.
The net ionic equation for the above reaction follows:
\(2H^+(aq)+2F^-(aq)+Ba^{2+}(aq)+2OH^-(aq)\rightarrow BaF_2(s)+2H_2O(l)\)
A solution contains 0.0400 M Ca2+ and 0.0990 M Ag+. If solid Na3PO4 is added to this mixture, which of the phosphate species would precipitate out of solution first? Ca3(PO4)2
Ag3PO4
Na3PO4
When the second cation just starts to precipitate, what percentage of the first cation remains in solution?
Answers
When the second cation first starts to precipitate, 80.8% of Ca²⁺ will still be in solution.
What is cation?A cation is an ion with a positive charge. It is formed when an atom loses one or more of its electrons, resulting in a net positive charge. Cations are attracted to anions (ions with a negative charge) due to their opposite charges. Cations are found in many different substances, including acids, bases, and salts.
Ca₃(PO₄)₂ will be the first species that separates out of solution when solid Na₃PO₄ is introduced to the mixture. This is due to Ca3(PO4)2 having a substantially lower solubility than Ag₃PO₄ and Na₃PO₄.
The proportion of the first cation (Ca²⁺ ) still in solution when the second cation (Ag⁺) is just beginning to precipitate will depend on the starting concentrations of the two cations. In this instance, the starting concentrations of Ca²⁺ and Ag⁺ are 0.0400 M and 0.0990 M, respectively. Therefore, 80.8% of Ca²⁺ will still be in solution when its second cation first begins to precipitate.
To learn more about cation
https://brainly.com/question/30754382
#SPJ1
Someone help i need an answer quick

Answers
Claim 1: Yes, there probably will be a lunar eclipse of the moon of Kepler-47c during a year.
Claim 2: No, there probably won't be a lunar eclipse of the moon of Kepler-47c during a year.
What are the evidence to support this claim?Kepler-47c is a moon orbiting Kepler-47 AB, a binary star system. This implies that the moon's light varies on a regular basis as it circles the two stars. These changes in light might result in a lunar eclipse.
Lunar eclipses occur when the moon passes through the Earth's shadow. While Kepler-47c circles two stars, it is still subject to Earth's gravitational pull and may pass into its shadow.
Lunar eclipses occur on a regular basis, with two to four eclipses occurring on average per year. Given the frequency of lunar eclipses, Kepler-47c is likely to see at least one lunar eclipse every year.
Evidence to support this claim 2:
Lunar eclipses occur when the moon passes through the Earth's shadow. While Kepler-47c is in the Earth's gravitational field, it is not on the same orbital plane as the Earth.
While Kepler-47c circles two stars, its orbit is not known to be stable. Variations in Kepler-47c's orbit may reduce the likelihood of a lunar eclipse.
While lunar eclipses are common, they are not uniformly distributed throughout the year. The locations and motions of the Earth, moon, and sun govern the timing and frequency of lunar eclipses.
Find out more on Kepler-47c here: https://brainly.com/question/28741788
#SPJ1
Which of the following compounds contains the greatest percent by mass of nitrogen?
(A) NH3
(B) HCN
(C) N2O
(D) NI3
Answers
NH3 contains the greatest percent by mass of nitrogen.
What is nitrogen?
All living things require the element of nitrogen. It is a colorless, odorless and tasteless gas that makes up 78% of the Earth's atmosphere. Nitrogen is found in all organic molecules, including proteins, nucleic acids, and other essential molecules for life. Nitrogen is essential for plant growth and is a key component of the nitrogen cycle. Nitrogen is important to the production of fertilizers and is used in industrial processes such as steel making and welding. Nitrogen also plays an important role in the environment, as it is part of the ozone layer and helps to reduce the amount of UV radiation that reaches the Earth's surface. Nitrogen is also used in many medical and pharmaceutical products, such as anesthetics and chemotherapy drugs
To learn more about nitrogen
https://brainly.com/question/1380063
#SPJ4
During electrolysis, where do reduction reactions occur?
Answers
Answer:
at the negative cathode
Explanation:
Reduction happens at the negative cathode because this is where positive ions gain electrons. Oxidation happens at the positive anode because this is where negative ions lose electrons.
Answer:
B. in the cathode of an electrolytic cell
Explanation:
Question 1
Given the equation: Q = mcAT
Q = heat (in Joules)
m = mass (in grams)
C = 4.18 (specific heat capacity)
AT change in temperature (°C)
How many Joules of heat energy are absorbed when 200 grams of water are heated from 20 C to 60 C.

Answers
The amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
To find the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C, we can use the equation Q = mcAT.
First, we need to find the value of m, which is the mass of the water in grams. In this case, it is given as 200 grams.
Next, we need to find the value of AT, which is the change in temperature in degrees Celsius.
This can be calculated by subtracting the initial temperature from the final temperature, which gives us 60 C - 20 C = 40 C.
The specific heat capacity of water, C, is given as 4.18 Joules per gram per degree Celsius.
Now we can plug in the values into the equation:
Q = mcAT
Q = (200 g) x (4.18 J/g°C) x (40°C)
Q = 33,440 J
Therefore, the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
for more such question on heat energy
https://brainly.com/question/25603269
#SPJ8
What was earth’s surface like? Landmasses? First land plants
Answers
Answer:
During the early Paleozoic Era, the Earth's surface was very different from what it is today. The continents were arranged differently, forming one large supercontinent called Pangea. This landmass was surrounded by a single large ocean called Panthalassa. The climate was much warmer and wetter than it is today, with no ice caps at the poles.
The first land plants, known as bryophytes, appeared during the early Silurian Period, around 430 million years ago. These plants were small and simple, lacking roots and vascular tissue. They grew in damp environments, such as along the edges of lakes and streams. They were important in the development of soils and in the colonization of land by other organisms, such as insects and other arthropods.
2. How many orbitals are in the following sublevels?
a. ls
b. 5s
c. 4d
d. 4f
e. 7s
f. 3p
Answers
2KBr + Cl₂ → 2KCI + Br₂
What type of chemical reaction is this?
Answers
Answer:
Displacement reaction
Explanation:
A displacement reaction is a reaction where a more reactive halogen will displace a less reactive one.
Reactivity decreases down group 17. So Chlorine, being the more reactive halogen, substitutes in the place of Br which is less reactive.
Magnesium nitride, Mg3N, undergoes a thermo decomposition reaction to form magnesium metal and nitrogen gas.
a. True
b. False
Answers
Answer:
a. True
Explanation:
Magnesium nitride, Mg₃N, is a binary salt. Upon heating, it decomposes into magnesium metal and nitrogen gas. The unbalanced equation is:
Mg₃N(s) ⇒ Mg(s) + N₂(g)
We can balance it with the trial and error method. First, we will balance N atoms by multiplying Mg₃N by 2.
2 Mg₃N(s) ⇒ Mg(s) + N₂(g)
Then, we get the balanced equation by multiplying Mg by 6.
2 Mg₃N(s) ⇒ 6 Mg(s) + N₂(g)
What mass of nitrogen trifluoride can be produced when 32.0 G of fluorine is reaction according to the table below
N2+3F2= 2NF3
Answers
When 32.0 g of F₂ react with enough N₂, 39.86g of NF₃ are formed.
What is stoichiometry?Stoichiometry is the study of the quantitative relationships or ratios between two or more substances undergoing a physical change or chemical change.
Let's consider the following balanced equation.
N₂ + 3 F₂ ⇒ 2 NF₃
To calculate the mass of NF₃ formed from 32.0 g of F₂, we need to consider the following relationships.
The molar mass of F₂ is 38.00 g/mol.The molar ratio of F₂ to NF₃ is 3:2.The molar mass of NF₃ is 71.00 g/mol.\(32.00gF_2 \frac{1molF_2}{38.00gF_2} \frac{2molNF_3}{3molF_2} \frac{71.00gNF_3}{1molNF_3} = 39.86gNF_3\)
When 32.0 g of F₂ react with enough N₂, 39.86g of NF₃ are formed.
Learn more about stoichiometry here: https://brainly.com/question/16060199
Which bonds form in the reaction shown in the diagram? 2 2H, + 0 H-H O=0 H-H → 2H 0 H-0-H H-OH A. The bonds between the two hydrogen atoms and between the two oxygen atoms B. The bonds between the two hydrogen atoms C. The bonds between the oxygen and hydrogen atoms D. The bonds between the two oxygen atoms

Answers
The water molecule is formed by the covalent bonding between oxygen and hydrogen atoms. Therefore, option C is correct.
What is covalent bonding ?Covalent bonding is a type of chemical bonding that involves the sharing of electrons between two atoms. In covalent bonding, the two atoms share a pair of electrons to fill their outermost electron shell and form a stable molecule.
This type of bonding usually occurs between non-metal atoms, which have a high electronegativity and tend to attract electrons strongly. In a covalent bond, the shared electrons are attracted to the positively charged nuclei of both atoms, creating a strong bond.
The strength of the bond depends on the number of shared electrons and the distance between the nuclei. Covalent bonds can be either polar or nonpolar, depending on the electronegativity difference between the two atoms.
In water the bond is formed between oxygen atom and two hydrogen atoms. Hence, C is correct.
Find more on covalent bonding:
https://brainly.com/question/12661797
#SPJ7
Al^+3
protons =
electrons =
neutrons =
N^-3
protons =
electrons =
neutrons =
Answers
Explanation:
aluminium
proton= 13
electron=13
neutron=27
nitrogen
proton= 7
electron=7
neutron= 14