a 500. gram iron ore sample was determined to contain 242 grams of iron. what is the mass percent of iron in the ore? a 500. gram iron ore sample was determined to contain 242 grams of iron. what is the mass percent of iron in the ore? 51.6 48.4 32.6 93.7 none of the above
Answers
242 grams more iron were found to be present in a 500 gram sample of iron ore. 48.4 mass percentage of iron is present in the ore.
To get the mass percent of a component of a compound, divide the result by 100 after dividing the mass of the component in 1 mole of a complex by the compound's molar. Although certain ores include quite so much as 66 % iron, the majority are in the 50–60 %, making deposits with less that 30 % iron commercially unappealing. The other components of an ore, referred to as gangue as a whole, can also affect the quality of the ore. 60% to 70% of haematite is made entirely of iron ore. 40% to 60% of limonite is pure iron. Siderite only contains 40% - 50% pure iron and is heavily contaminated. Iron ore in haematite is 60–70% pure iron. In limonite, there is 40–60% pure iron. Siderite has several impurities.
Learn more about iron
https://brainly.com/question/1626377
#SPJ4
Related Questions
A nurse is collecting a stool specimen of a client suspected of having clostridium difficile. Which guideline is recommended for this procedure?
Answers
The guideline that is recommended for a nurse who is collecting a stool specimen of a client suspected of having clostridium difficile includes the following procedures:
1. Use a new pair of gloves for each step in the process of specimen collection, removing the old gloves each time.
2. A bedpan is provided by the nurse for the client to have a bowel movement.
3. The bedpan is then thoroughly washed, disinfected, and dried.
4. The client's buttocks are washed with warm, soapy water and dried with a clean towel.
5. The nurse wears gloves to remove the stool specimen from the bedpan.
6. The specimen is placed in a sterile container and taken to the laboratory as soon as possible.
7. The nurse takes care to avoid touching any part of the specimen container that will come in contact with the laboratory personnel.
The nurse should adhere to standard infection control procedures to protect both the nurse and the client from potential infection. The nurse should make certain to wash their hands before and after the procedure. A stool sample is usually the best way to confirm the diagnosis of Clostridium difficile infection. It can be challenging to diagnose C. difficile infection in the early stages.
To know more about clostridium difficile visit
https://brainly.com/question/13552507
#SPJ11
Ammonia chemically reacts with oxygen gas to produce nitric oxide and water. What mass of oxygen gas is consumed by the reaction of of ammonia?.
Answers
15.90 g mass of oxygen gas is consumed by the reaction of of ammonia. when Ammonia chemically reacts with oxygen gas to produce nitric oxide and water.
The mole is the fundamental unit of measurement in SI units for the amount of substance. It is defined as the mass of substance containing in the same number of particles found in 12.000 g of carbon atom (12C).
The molar mass is defined as the sum of masses of the atoms or molecules expressed in grams, and it is contributed by one mole of an atom or molecule.
The significant figures of a number are digits that carry meaning contributing to their measurement resolutions. The number of significant figures indicates the precision of a measurement instead of its magnitude. There are certain rules to determine significant digit.
The number of moles is calculated by the formula as follows:
Number of moles = given mass(g/molar mass(g/mol)
Step: 1
The reaction is as follows:
4NH3+5O2⟶4NO+6H2O
Here, the reactants are ammonia and oxygen and the product formed include nitric oxide and water. More precisely we can say that, 4 moles of ammonia react with 5 moles of oxygen to give 4 moles of nitric oxide and 6 moles of water.
Hint:
Find the mass of oxygen gas.
Step: 2
Consider the reaction as follows
4NH3+5O2⟶4NO+6H2O
Weight of 4 moles of ammonia = 4×17
=68g
Weight of 5 moles of oxygen = 5×32
=160g
Here
4 moles of ammonia ≡ 5 moles of oxygen
Therefore:
68 g of ammonia ≡ 10 g of oxygen
Therefore,
The mass of oxygen corresponding to 6.76 g of ammonia is calculated as follows:
mass of oxygen = 6.6g×160g / 68g
=15.90 g
Initially, the chemical reaction is considered. From that, it is clear that
4 moles of ammonia ≡ 5 moles of oxygen
Therefore,
68 g of ammonia ≡ 160 g of oxygen
Then, the mass of oxygen corresponding to 6.76g of ammonia is calculated by multiplying 6.76g with 160 g of oxygen and divided by the answer with 68 g. The mass of oxygen corresponding to 6.76g of ammonia is 15.90 g
The mass of oxygen corresponding to 6.76 g of ammonia is 15.90 g.
Learn more about Oxygen here:
https://brainly.com/question/13370320
#SPJ4
Which one of the following salts when dissolved in water will hydrolyse?
Answers
Option c) NH4Cl will hydrolyze when dissolved in water.
Hydrolysis is a chemical reaction that occurs when a salt reacts with water, resulting in the formation of an acidic or basic solution. In this case, when NH4Cl (ammonium chloride) is dissolved in water, it undergoes hydrolysis.
The ammonium ion (NH4+) is the conjugate acid of ammonia (NH3), which is a weak base. When NH4Cl dissociates in water, the ammonium ion reacts with water to form NH3 and H3O+ (hydronium ion). This process is called hydrolysis.
NH4+ (aq) + H2O (l) ↔ NH3 (aq) + H3O+ (aq)
The formation of NH3 leads to an increase in the concentration of hydroxide ions (OH-) in the solution, making it slightly basic. At the same time, the presence of H3O+ ions makes the solution slightly acidic. Therefore, the hydrolysis of NH4Cl results in a slightly acidic and slightly basic solution.
In contrast, salts like NaCl and KCl do not undergo hydrolysis when dissolved in water because they consist of cations (Na+ and K+) and anions (Cl-) that do not react with water to form acidic or basic species.
Na2SO4 (sodium sulfate) is also an example of a salt that does not undergo hydrolysis. The sulfate ion (SO42-) does not react with water to form acidic or basic species, so the solution remains neutral.
Therefore, among the given options, only NH4Cl undergoes hydrolysis when dissolved in water.
Know more about Hydrolysis here:
https://brainly.com/question/30468294
#SPJ8
The question is incomplete. Find the full content below:
Which one of the following salts when dissolved in water will hydrolyse?
a) NaCl
b) KCl
c) NH4Cl
d)Na2SO4
An investigation involves determining which metal is better for making pots that will cook food faster.
Answers
A resistance of 60Ω has a current of 0.73A through it when it is connected to the terminals of a battery. The voltage of the battery is ______V. (Just write the number.
Answers
Answer: 43.8 Volts
Explanation:
Ohms law states that current is directly proportional to voltage applied.
\(V=I\times R\)
where V = voltage = ?
I = current = 0.73 A
R = resistance = 60 ohm
Putting in the values we get:
\(V=0.73A\times 60ohm=43.8V\)
Thus the voltage of the battery is 43.8 Volts
What happens when an electrically charged pencil is placed close to the water running from a faucet?
the pencil attracts the water
the pencil becomes positively charged
the water attracts the pencil
the water repels the pencil
Answers
Answer:
the water repels the pencil
Explanation:
Since the an electrically charged pencil is placed close to the water running from a faucet :D
Answer:
Option 2
Explanation:
:)
What is the formula for S2O5
Answers
Answer:
chemical formula
Explanation:
The limiting reactant, O2, can form up to 2.7 mol Al2O3. What mass of Al2O3 forms?
Al2O3 : 101.96 g/mol
[?] g Al₂O3
Answers
Answer:
280 g Al₂O₃
Explanation:
To find the mass, you need to multiply the given value by the molar mass. This will cause the conversion because the molar mass exists as a ratio; technically, the ratio states that there are 101.96 grams per every 1 mole Al₂O₃. It is important to arrange the ratio in a way that allows for the cancellation of units. In this case, the desired unit (grams) should be in the numerator. The final answer should have 2 sig figs to reflect the given value (2.7 mol).
Molar Mass (Al₂O₃): 101.96 g/mol
2.7 moles Al₂O₃ 101.96 g
------------------------ x ------------------- = 275 g Al₂O₃ = 280 g Al₂O₃
1 mole
*
Identify the element found in the f-block.
Cm
K
W
Br
Ag
Answers
AgNo3.
Electrolysis happening in water.
5.6l of Oxigen goes in Anod how much Ag goes on Ch?
Answers
Which statement best explains the reason that the metal part of a seatbelt is a better thermal conductor than the plastic part?
A. The metal has densely packed particles, which limits energy transfer by the collision of particles.
B. The metal has densely packed particles, which allows for more collisions in a given area.
C. The metal has loosely packed particles, which limits energy transfer by the collision of particles.
D. The metal has loosely packed particles, which allows for more collisions in a given area.
Answers
Answer:
b it seems like the only one that makes sense
the correct answer is b, I just took the quiz and the other answer on this question was correct
Which of the following is a possible way to describe the SO3 component in the reaction below?
S8 (s) + 1202(g) → 8SO3(g)
8 molecules SO3
8 atoms SO3
80.07 g SO3
32 L SO3
Answers
The possible way to describe the \(SO_3\) component in the reaction would be 8 molecules or 8 atoms of \(SO_3\), Options 1 and 2.
State of matterEach component of a reaction has its state. Recall that matters are anything with weight and occupies space.
In the reaction: \(S_8 (s) + 12O_2(g) -- > 8SO_3(g)\)
Eight moles of sulfur react with 12 moles of oxygen molecules to produce 8 moles of sulfur (III) oxide. According to the reaction:
The state of sulfur is solid (s)The state of oxygen is gas (g)The state of sulfur (III) oxide is gas (g).The amount of gas can be described in terms of its number of molecules and its number of atoms. Thus, either 8 molecules or 8 atoms of sulfur (III) oxide can be used to describe the amount of \(SO_3\) produced in the reaction.
More on states of matters can be found here: https://brainly.com/question/29476563
#SPJ1
1. State the general period and group trends among
main-group elements with respect to each of the
following properties:
a. atomic radii
b. first ionization energy
c. electron affinity
d. ionic radii
e. electronegativity
2. a. In general, how do the periodic properties of
the d-block elements compare with those of
the main-group elements?
b. Explain the comparison made in (a).
3. For each main-group element, what is the
relationship between its group number and
the number of valence electrons that the group
members have?
Answers
Explanation:
ok so I believe:
a. increase in atomic radii going down a group and decrease going across a period.
b. increase in IE1 going across a period and up a group. the highest IE1 elements are like He, Ne, F and first ionization energy decreases moving further away from those elements moving south west on the periodic table.
c. electron affinity increases moving across a period left to right.
d. ionic radii increases going down a group and decreases going across a period left to right
e. electronegativity increases across a period but decreases down a group. (opposite for electropositivity)
what is meant by the d-block elements? are you referring to the spdf location?
it is for science class please help answer the questions and fast . I will
mark brainliest but it has to be right.

Answers
Bioluminescence Imaging.
Explanation:
Bioluminescence is appealing as an approach for in vivo optical imaging in mammalian tissues because these tissues have low intrinsic bioluminescence; therefore, images can be generated with remarkably high signal-to-noise ratios. A variety of different bioluminescent systems have been identified in nature, each requiring a specific enzyme and substrate. Although the most commonly used bioluminescent reporter for research purposes has been luciferase from the North American firefly (Photinus pyralis; FLuc), useful luciferases have also been cloned from jellyfish (Aequorea), sea pansy (Renilla; RLuc), corals (Tenilla), click beetle (Pyrophorus plagiophthalamus), and several bacterial species (Vibrio fischeri, V. harveyi).
Hope this is what you need!
Please give Brainliest!
Question 3, please help!! 10 points

Answers
Answer:
0.68 centiseconds
Explanation:
I looked it up
Answer:
The person above is correct 6800 converted to centiseconds is .68
Explanation:
you have to divide x by 10000 to convert it into centiseconds
in this case x=6800
6800÷10000= 0.68
Will the energy released in the synthesis of water equal the energy absorbed in the decomposition of water?
Answers
Answer:
yes
Explanation:
the energy released in the synthesis of water is equal to the energy absorbed in the decomposition of water. This outcome happens because the difference in energy between the reactants and the products in both cases is equal. . It follows the law of conservation of energy.
how much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
helpppppppppppp
please

Answers
NEUTRON: Neutral charge. Relative mass of 1. Found in the nucleus.
ELECTRON: Negative (-) charge. Relative mass of .0005. Found on the outside of the nucleus.
Chadwick proved the existence of neutrons - elementary particles devoid of any electrical charge.
Rutherford discovered alpha and beta rays and proposed the mass of radioactive decay. Postulated the nuclear structure of the atom.
J.J Thompson proposed the plum pudding model of the atom. Showed that all atoms contain tiny negatively charged subatomic particles or electrons.
David says, "Pure honey has nothing else added." Susan says, "The honey is not really pure. It is a mixture of many different substances." Who is right? Explain your answer.
Answers
Answer:
Susan is right
Explanation:
Honey is a natural substance extracted from bees. Like every other natural substance, honey is a mixture of many different substances. An analysis of the so called 'pure honey' will reveal that many chemical species compose the natural substance called honey.
Hence Susan is right when she says that honey is not really pure but a mixture of substances.
Of the compounds shown, which molecule would be named trans-4-methylpent-2-ene?
A. C
B. D
C. A
D. B

Answers
Answer:
the answer would be B
Explanation:
Answer:
B. D I got it right on the test.
give three examples of giant covalent substances
Answers
Calculate the concentration of each solution in mass percent.
Part A
103 g KCl in 628 g H2O
Part B
30. 3 mg KNO3 in 9. 29 g H2O
Part C
9. 18 g C2H6O in 72. 2 g H2O
Answers
The concentration 103 g \(KCl\) in 628 g \(H_2O\) is 14.1% by mass
The concentration 30. 3 mg \(KNO_3\) in 9. 29 g \(H_2O\) is 0.325% by mass.
The concentration 9. 18 g \(C_2H_6O\) in 72. 2 g \(H_2O\) is 11.3% by mass.
To calculate the concentration of a solution in mass percent, we need to determine the mass of the solute and the mass of the solution. The mass percent is then calculated as:
Mass percent = (Mass of solute / Mass of solution) x 100%
Part A:
Mass of \(KCl\)= 103 g
Mass of \(H_2O\) = 628 g
Mass of solution = Mass of \(KCl\) + Mass of \(H_2O\) = 103 g + 628 g
= 731 g
Mass percent of \(KCl\) = (103 g / 731 g) x 100% = 14.1%
Therefore, the concentration of the \(KCl\) solution is 14.1% by mass.
Part B:
Mass of \(KNO_3\) = 30.3 mg = 0.0303 g
Mass of \(H_2O\) = 9.29 g
Mass of solution = Mass of \(KNO_3\) + Mass of \(H_2O\) = 0.0303 g + 9.29 g
= 9.3203 g
Mass percent of \(KNO_3\) = (0.0303 g / 9.3203 g) x 100%
= 0.325%
Therefore, the concentration of the \(KNO_3\) solution is 0.325% by mass.
Part C:
Mass of \(C_2H_6O\) = 9.18 g
Mass of \(H_2O\) = 72.2 g
Mass of solution = Mass of \(C_2H_6O\) + Mass of \(H_2O\) = 9.18 g + 72.2 g
= 81.38 g
Mass percent of \(C_2H_6O\) = (9.18 g / 81.38 g) x 100%
= 11.3%
Therefore, the concentration of the \(C_2H_6O\) solution is 11.3% by mass.
Learn more about Mass percent at
brainly.com/question/5394922
#SPJ4
state the names related to climate conditions for various crops and state their requirements. state the types of crops based on any of the factors.
Answers
Solar radiation, temperature, and rainfall are the three main climatic elements that affect how quickly plants grow, develop, and produce food. The buildup of dry materials must occur at the ideal temperature.
Solar radiation, temperature, and rainfall are the three main climatic elements that affect how quickly plants grow, develop, and produce food. The buildup of dry materials must occur at the ideal temperature. The following are some of the ideal circumstances for strong plant growth: Air temperature: 24 °C Day/ 19 °C Day (75F/65F) H2O temperature: cold at 26 °C, hot at 24 °C, not to exceed 25 °C. Minimum of 50 and no more than 70% relative humidity These fundamental resources for the food-producing industry, according to Perrin, include land, water, and other natural resources as well as important aspects like climate and ecological resilience. Environmental elements include air, water, soil, climate, native plants, and landforms. By definition, environmental factors have an impact on daily life and are a significant contributor to the disparities in health that exist between different geographic regions.
Learn more about Solar radiation here:
https://brainly.com/question/23338147
#SPJ4
How much energy is released when a peanut is burned in a calorimeter? The temperature of 1.00Kg of water rose from 22.6°C to
26.5°C. The specific heat of water is 4.184J/g °C.
Answers
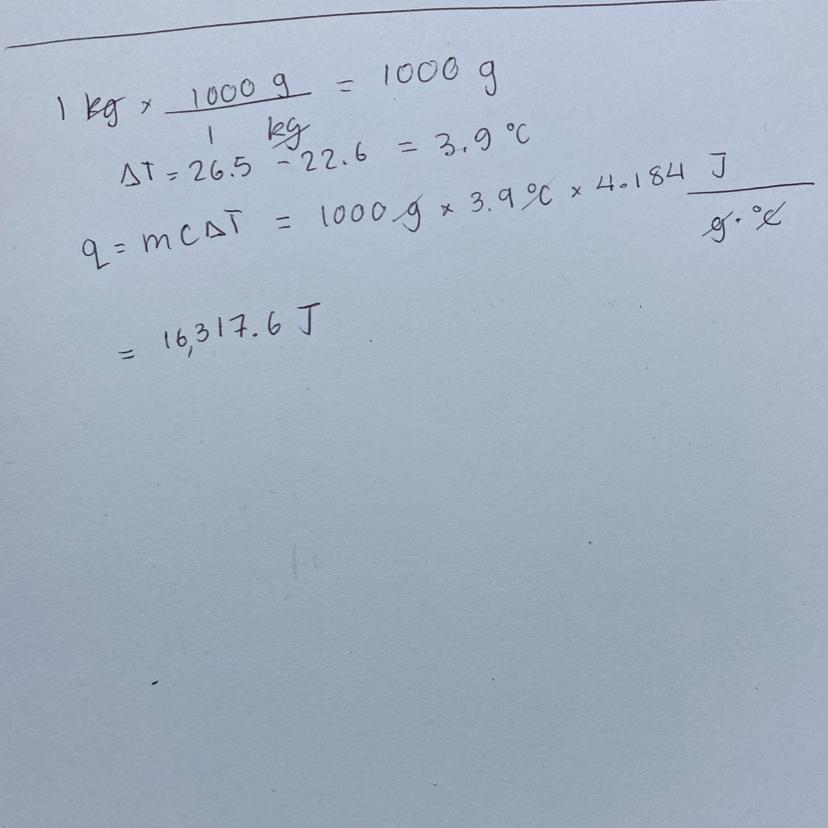
A generator can produce both _______________________ (DC), which flows in one direction,and AC current;large power plants produce ___________________.
Answers
Answer:
1. direct current. 2. electrical power
Explanation
i believe number 2 is right
2-propanol had a _____a_____ δt value compared to 1-propanol because _____b____
Answers
2-propanol had a lower δt value compared to 1-propanol because of its different molecular structure.
The difference in δt values between 2-propanol and 1-propanol can be attributed to the position of the hydroxyl group (-OH) in the molecule. In 2-propanol, the hydroxyl group is attached to the middle carbon atom, while in 1-propanol, it is attached to the terminal carbon atom.
This difference in molecular structure results in varying intermolecular forces, leading to different boiling points and evaporation rates. 2-propanol has stronger intermolecular forces due to the increased branching, which means it evaporates more slowly and has a lower temperature change (δt) value.
The δt value of 2-propanol is lower than that of 1-propanol because its molecular structure creates stronger intermolecular forces, resulting in a slower evaporation rate and a smaller temperature change.
For more information on molecular structure kindly visit to
https://brainly.com/question/29857692
#SPJ11
In test tube 3, 3 mL of .002 M Fe(III) solution is combined with 7 mL of .02 M 5-sulfosaliclic acid (SSA) solution. What is the mole fraction of SSA in the test tube
Answers
Answer:
0.96
Explanation:
Number of moles of Fe(III) = 3/1000 * 0.002 M = 6 * 10^-6 moles
Number of moles of SSA = 7/1000 * 0.02 = 1.4 * 10^-4 moles
Total number of moles = 6 * 10^-6 moles + 1.4 * 10^-4 moles
Mole fraction of SSA = number of moles of SSA/ total number of moles.
Mole fraction of SSA = 1.4 * 10^-4 moles/ 6 * 10^-6 moles + 1.4 * 10^-4 moles
Mole fraction of SSA = 0.96
If an object increases in speed, it must be as a result of
o increasing friction.
o unbalanced forces.
O gravitational attraction.
O air resistance.
please hurry i’m taking a test
Answers
Answer:
a; increasing fraction
Explanation:
Calcium carbonate (CaCO3) is an important component of coral reefs. How many moles are in 98.6 g of CaCO3? Type in your answer using the correct number of significant figures.
98.6 g CaCO3 =
mol CaCO3
Answers
Calcium carbonate (CaCO3) is an important component of coral reefs. 0.986 moles are in 98.6 g of CaCO3.
What do you mean by mole ?The term mole is defined as the amount of substance of a system which contains as many elementary entities.
One mole of any substance is equal to 6.023 × 10²³ units of that substance such as atoms, molecules, or ions. The number 6.023 × 10²³ is called as Avogadro's number or Avogadro's constant.
Number of moles of CaCO3 = Mass / Molar mass
Number of moles of CaCO3 = 98.6 g / 100g/ mol
= 0.986 moles
The balanced chemical equation of this reaction is as follows:
CaCO₃ → CaO + CO₂
Therefore, the reaction ratio is 1:1:1.
So, 0.986 moles of CaO are formed.
Thus, 98.6 g CaCO3 = 0.986 moles CaCO3.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ1
TNT has relatively small energy per pound. However, it is a very effective explosive. Why?
Answers
TNT (Trinitrotoluene) has relatively small energy per pound, but it is a very effective explosive due to the following reasons:
1. It is an insensitive explosive: TNT has a high ignition temperature, making it less prone to accidental detonation. TNT can also resist shock and friction, making it a stable explosive.
2. High detonation velocity: TNT is capable of detonating at a speed of 6,900 m/s. This high velocity allows TNT to produce a supersonic shockwave that can cause significant damage to its surroundings.
3. High gas yield: When TNT explodes, it produces a large amount of gases, which further increases the pressure exerted on its surroundings. This high-pressure shockwave causes significant damage to buildings and structures.
4. Easy to manufacture: TNT is relatively easy and cheap to manufacture, making it a popular explosive for military and industrial applications.
To know more about ignition temperature, visit:
https://brainly.com/question/31030276
#SPJ11