A 150 ml solution of naoh is titrated with 3. 0 m hi. It takes 75 ml of the titrant to reach the endpoint, shown when bromthymol blue changed from blue to green. As you continued adding hi past the endpoint you saw the solution turn yellow. What is the ph of the original naoh solution?.
Answers
The pH of the original NaOH solution is 13.
Given, the volume of NaOH solution = 150 mL, The concentration of HI = 3.0 M, The volume of HI required to reach the endpoint = 75 mL, The volume of NaOH solution = 150 mL, Therefore, the number of moles of HI used = (3.0 mol/L) x (75 mL/1000 mL) = 0.225 mol.
Similarly, the number of moles of NaOH = 0.225 mol (NaOH and HI react in 1:1 mole ratio). Since the volume of NaOH solution is 150 mL, the concentration of NaOH is given by = (0.225 mol) / (150 mL/1000 mL) = 1.5 M. The pOH of the NaOH solution is given by = -log[OH-]pOH = -log[OH-]pOH = -log[1.5]pOH = 0.176. The pH of the NaOH solution is given by: pH = 14 - pOHpH = 14 - 0.176pH = 13. Hence, the pH of the original NaOH solution is 13.
Learn more about moles here:
https://brainly.com/question/30885025
#SPJ11
Related Questions
If I have one mole of sulfur, how many atoms would that be?
Answers
Answer:
Atoms of sulfur = 9.60⋅g32.06⋅g⋅mol−1×6.022×1023⋅mol−1
Explanation:
because the units all cancel out, the answer is clearly a number, ≅2×1023 as required.
What kind of industrial facilities release Nitrous Oxide?
Answers
Answer:
the agriculture sector is an industrial facility that releases nitrous oxide
Explanation:
Answer: Hello!
Agriculture sector. The agriculture sector dominates emissions of N2O: emissions from agricultural soils in 2019 account for 55% of total UK emissions, and other agricultural sources add another 12%.
Explanation:
Feel Free to ask me anything if questions. Mark me brainest please. Hope I helped! Hope you make an 100%
-Anna♥
Urgent help please!!

Answers
Answer:
1. 2.1 moles of Mg
2. 0.72 mole of Mg(OH)2
Explanation:
1. We'll begin by writing the balanced equation for the reaction. This is given below:
3Mg + 2AlBr3 —> 3MgBr2 + 2Al
From the balanced equation above, 3 moles of Mg reacted to produce 2 moles of Al.
Therefore, Xmol of Mg will react to produce 1.4 moles of Al i.e
Xmol of Mg = (3 x 1.4)/2
Xmol of Mg = 2.1 moles.
Therefore, 2.1 moles of Mg is required to 1.4 moles of Al.
2. We'll begin by calculating the number of mole in 26g of water, H2O.
This is illustrated below:
Molar mass of H2O = (2x1) + 16 = 18g/mol
Mass of H2O = 26g
Number of mole of H2O =?
Mole = Mass /Molar Mass
Number of mole of H2O = 26/18
Number of mole of H2O = 1.44 moles
Next, we shall write the balanced equation for the reaction. This is given below:
2HNO3 + Mg(OH)2 —> Mg(NO3)2 + 2H2O
Finally, we can obtain the number of mole of Mg(OH)2 used in the reaction as follow:
From the balanced equation above,
1 mole of Mg(OH)2 reacted to produce 2 mole of H2O.
Therefore, Xmol of Mg(OH)2 will react to produce 1.44 moles of H2O i.e
Xmol of Mg(OH)2 = (1 x 1.44)/2
Xmol of Mg(OH)2 = 0.72 mole.
Therefore, 0.72 mole of Mg(OH)2 was used in the reaction.
Which of the following items describe a mole? a. Avogadro's number of items b. 6.022 multiply 10^23 items c. mass multiply acceleration d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon.
Answers
The correct descriptions of a mole are:
a. Avogadro's number of items
b. 6.022 × \(10^2^3\) items
d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon.
a. Avogadro's number of items:
Avogadro's number is a fundamental constant in chemistry and is defined as the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. It is approximately equal to 6.022 × \(10^2^3\) items. Therefore, option a correctly describes a mole as Avogadro's number of items.
b. 6.022 × \(10^2^3\) items:
This is the numerical value of Avogadro's number. As mentioned earlier, it represents the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. So, option b is another correct description of a mole.
c. Mass multiplied by acceleration:
This description does not accurately describe a mole. The product of mass and acceleration is a measure of force (Newton's second law of motion) and is unrelated to the concept of a mole in chemistry.
d. The amount of a substance containing the same number of formula units as there are atoms in 12 g of carbon:
This is a correct description of a mole. It refers to the concept of the molar mass, where one mole of a substance contains the same number of particles (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This concept allows for the conversion between mass (in grams) and the number of moles.
So, the correct options are a, b, and d.
To know more about Avogadro's number, refer
here:https://brainly.com/question/28812626
#SPJ4
Substance A is a liquid at room temperature and pressure, while substance B is a gas under the same conditions. Both are molecular substances. Based on this observation, we can say that the intermolecular attractions in substance A are __________ those in substance B.
A. Stronger than
B. The same strength as
C. Weaker than
Answers
Substance A is liquid at room temperature and pressure, while substance B is gas under the same conditions.Based on this observation, we can say that intermolecular attractions in substance A are stronger than those in substance B.
What are intermolecular attractions?Intermolecular attractions is a force by which atoms in a molecule combine. it is basically an attractive force in nature. It can act between an ion and an atom as well.It varies for different states of matter that is solids, liquids and gases.
The forces of attraction are maximum in solids as the molecules present in solid are tightly held while it is minimum in gases as the molecules are far apart . The forces of attraction in liquids is intermediate of solids and gases.
Learn more about intermolecular attractions,here:
https://brainly.com/question/10626096
#SPJ1
How much energy is required to raise the temperature of 3 kg of lead from 15
°C to 20°C? Use the table below and this equation: Q = mc&T.

Answers

30.) What charge would you expect from a Group 16 atom when it becomes an ion?
a.) 1-
b.) 2+
c.) 1+
d.) 2-
Answers
Explanation: atoms of group 16 gain two electrons and form ions with a 2− charge
need some help with just one question please help.What is the pH of a 9.7 x 10-5 M NaOH solution? (2 formulas)

Answers
If 4.0 mL of a 3.0 M HCl solution is exactly neutralized by a 3.0 mL sample of NaOH, then what is the concentration of the NaOH solution?

Answers
Answer:
B. 4.0
Explanation:
I need help please help me please

Answers
Answer: compound thing(picture attached)
Bronze is an alloy made by combining copper and tin. The exact composition of bronze can vary depending on the desired properties, but generally, bronze contains anywhere from 5% to 25% tin.
The reasons why the first humans experimented with making bronze are not fully known, but it is believed that they discovered that adding tin to copper improved its properties, making it harder, more durable, and easier to cast. This would have made it more suitable for weapons, tools, and other objects.
Bronze provided several benefits to early humans. Firstly, bronze tools and weapons were much more durable than those made of pure copper or stone. This made it easier for early humans to hunt, farm, and build, and allowed them to produce more sophisticated and efficient tools. Additionally, bronze objects were more aesthetically pleasing and could be used for decorative purposes. Bronze also played an important role in early trade, as it could be used as a form of currency and was highly valued by many cultures.
In summary, bronze was an important technological advancement in early human history, and its discovery and use played a significant role in the development of human civilization.
Explanation:
Side groups of amino acids are typically classified under which of the following?
A) polar, nonpolar
B) linear, circular
C) alpha, omega
D) long, short
E) primary, secondary
Answers
Side groups of amino acids are typically classified as A) polar or nonpolar.
Amino acids are the building blocks of proteins and are composed of an amino group (-NH2), a carboxyl group (-COOH), and a side chain that is unique to each amino acid. The side chains can be classified as either polar or nonpolar based on their chemical properties.
Polar side chains contain functional groups such as -OH or -NH2 that can participate in hydrogen bonding with water or other polar molecules. Examples of polar amino acids include serine (Ser), threonine (Thr), and asparagine (Asn).
Nonpolar side chains, on the other hand, are hydrophobic and do not interact with water or other polar molecules. These side chains are typically composed of hydrocarbons and can be further divided into aliphatic or aromatic categories. Examples of nonpolar amino acids include glycine (Gly), alanine (Ala), and phenylalanine (Phe).
In summary, side groups of amino acids are classified as polar or nonpolar based on their chemical properties.
So A is correct option.
For more questions like Proteins click the link below:
https://brainly.com/question/30986280]
#SPJ11
(i) Give the two dyes that are mixed to make brown hair dye.
Answers
Answer:
green n yellow and white and black and white sq
biomarkers such as serum micronutrient levels, which can determine a person's fruit and vegetable intake more accurately than can personal interviews, are used in which field of modern epidemiology?
Answers
Answer:
Biomarkers such as serum micronutrient levels are used in the field of nutritional epidemiology. Nutritional epidemiology is a subfield of epidemiology that focuses on the relationship between diet and health. Biomarkers are substances that can be measured in the body and that provide information about a person's health. Serum micronutrient levels can be used as biomarkers of fruit and vegetable intake because they are a reflection of the amount of fruits and vegetables that a person has eaten.
There are a number of advantages to using biomarkers to assess fruit and vegetable intake. First, biomarkers are more accurate than self-reported dietary intake. People often underestimate their fruit and vegetable intake, so biomarkers can provide a more accurate assessment of their diet. Second, biomarkers can be used to assess fruit and vegetable intake over time. This is important because fruit and vegetable intake is associated with a number of health outcomes, including a reduced risk of chronic diseases such as heart disease, stroke, and cancer.
Biomarkers such as serum micronutrient levels are a valuable tool for nutritional epidemiology. They can be used to assess fruit and vegetable intake more accurately than self-reported dietary intake, and they can be used to assess fruit and vegetable intake over time. This information can be used to develop interventions to improve fruit and vegetable intake and to reduce the risk of chronic diseases.
Explanation:
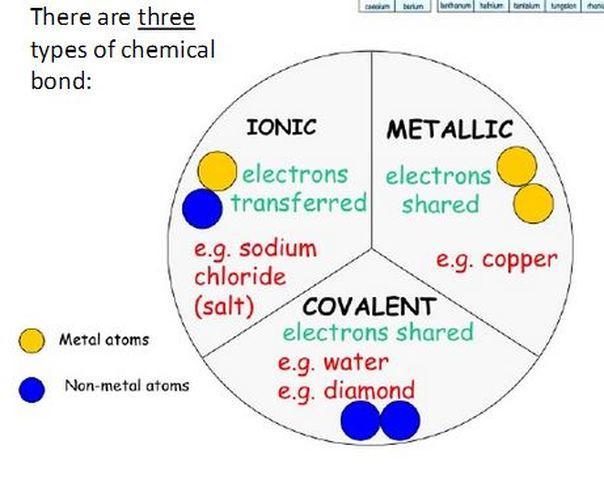
match these items. match the items in the left column to the items in the right column. 1 . ionic bond a chemical bond between atoms with similar electronegativities 2 . covalent bond a measure of the ability of an atom to attract electrons within a chemical bond 3 . metallic bond a bond between atoms of greatly differing electronegativities 4 . electronegativity the bond formed in metals, holding metals together
Answers
1. a chemical bond between atoms with similar electronegativities - covalent bond
2. a measure of the ability of an atom to attract electrons within a chemical bond - Electronegativity
3. a bond between atoms of greatly differing electronegativities - Ionic bond
4. the bond formed in metals, holding metals together - Metallic bond
A covalent bond is a bond formed by sharing electrons between two atoms that occur in the bond. It generally forms between atoms with similar electronegativity values.
An ionic bond is a bond formed between two oppositely charged ions of her and held by strong electrostatic attraction. It forms between atoms that have vastly different electronegativities.
Electronegativity is the tendency of an atom in a covalent bond to attract a shared pair of electrons.
A metallic bond is a bond formed by electrostatic attraction between a positively charged metal ion and a conduction electron.
learn more about chemical bond at https://brainly.com/question/3162660
#SPJ4
Volume of 22mm x 15 mm x 2.0 mm
Answers
The formula for the volume of a rectangular solid is Volume = length * width * height, or V = lwh.
\(volume = 22mm \times 15mm \times 2.0mm \\ = 22mm \times 30mm \\ = 660mm \\ \)Converting to cm = \( \frac{660}{10} = 66 {cm}^{3 } \\ \)
- BRAINLIEST answerer
The average person in the United States is exposed to the following amount of radiations annually. Rank the following source of radiation in the increasing order of their ability to cause harm to living tissue. Weapons-test fallout 1 millirem Cosmic radiation 26 millirems Air (radon-222) 0. 198 rems Diagnostic X rays 0. 040 rems Television tubes 11 millirems Nuclear medicine 0. 015 rems Ground 33 millirems Rank from highest to lowest. To rank items as equivalent, overlap them
Answers
To rank the sources of radiation in the increasing order of their ability to cause harm to living tissue based on the given annual exposure amounts, we can arrange them as follows:
Nuclear medicine: 0.015 rems
Diagnostic X-rays: 0.040 rems
Weapons-test fallout: 1 millirem
Television tubes: 11 millirems
Cosmic radiation: 26 millirems
Air (radon-222): 0.198 rems
Ground: 33 millirems
Ranking them from highest to lowest harm, we have:
Ground (33 millirems) ≈ Cosmic radiation (26 millirems)
Air (radon-222) (0.198 rems)
Weapons-test fallout (1 millirem)
Television tubes (11 millirems)
Diagnostic X-rays (0.040 rems)
Nuclear medicine (0.015 rems)
Please note that this ranking is based on the given annual exposure amounts and the relative potential harm to living tissue. The specific risks and effects of radiation exposure can vary depending on factors such as duration, type of radiation, and individual susceptibility.
Learn more about sources of radiation here:
https://brainly.com/question/14402170
#SPJ11
If there is no subscript on an element symbol there is/are __________________ atom(s) of that element.
Answers
Answer:
If there is no subscript written after a symbol that means there is one atom of that element present
Explanation:
correct me if I'm
If there is no subscript on an element's chemical symbol there is one atom of that element.
What is an element ?
It is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.Elements combine to form compounds.
Learn more about element,here:
https://brainly.com/question/24407115
#SPJ6
What electrical charges do these parts have ?
Answers
Answer:
which parts electrical charges you are asking ??
A medical researcher finds a high incidence of covalent bonds between thymine and other nucleotides in the dna of skin cells of a patient. The most likely cause is.
Answers
The reason behind the high incidence of covalent bonding between thymine molecules and other nucleotides is UV exposure.
What is thymine?Thymine is a pyrimidine base of the nucleic acid with6 membered ring containing alternate NH bonds. Thymine bonds hydrogen bond with the adenine base of the other strand of DNA. This hydrogen bonding between nitrogen bases make the DNA double stranded.
Upon UV exposure thymine tends to dimerize and the two adjacent thymine molecules links together by covalent bonds. This very unusual and abnormal to body and which cases mutation to DNA results in the inhibition of DNA replication.
Therefore, the cause of covalent bonding thymine and other nucleotides is UV exposure.
To find more on thymine, refer here:
https://brainly.com/question/907132
#SPJ1
A gas occupies a volume of 0.7 L at 10.1 kPa. What volume will the gas occupy at 101 kPa?
Answers
Answer:
7L
Explanation:
Divide 101 by 10.1(kPa) and you get 10.
10 x .7 = 7
The concept Boyle's law is used here to determine the new volume of gas . Boyle’s law was put forward by the Anglo-Irish chemist Robert Boyle in the year 1662. Here the new volume of the gas is 0.07 L.
What is Boyle's law?A gas law known as Boyle's law asserts that a gas's pressure is inversely proportional to its volume when it is held at a fixed temperature and of a given mass. To put it another way, as long as the temperature and volume of the gas remain constant, the pressure and volume of the gas are inversely proportional to one another.
For a gas, the relationship between volume and pressure (at constant mass and temperature) can be expressed mathematically as follows.
P = k*(1/V) ⇒ PV = k
For two different gases, the equation is:
P₁V₁ = P₂V₂
V₂ = P₁V₁ / P₂
10.1 × 0.7 / 101 = 0.07 L
To know more about Boyle's law, visit;
brainly.com/question/30367067
#SPJ2
What type of elements are likely to form ionic bonds and why
Answers
Answer:
An ionic bond is formed when a metallic element and a non-metallic element form a compound. Elements form ionic bond to gain more stability.
metallic calcium crystallized in a face-centered cubic lattice and the atomic radius of calcium is 1.97å. calculate the edge length, a, of a unit cell of calcium.
Answers
The edge length (a) of the unit cell of calcium in a face-centered cubic lattice is approximately 3.206 Å.
In face-centered cubic (FCC) lattice, there are 4 atoms per unit cell. The edge length, denoted as "a," can be calculated using the atomic radius.
In an FCC lattice, the body diagonal of the unit cell is equal to 4 times the atomic radius (2√2 × r). From this, we can relate the body diagonal length (BDL) to the edge length (a) using the Pythagorean theorem:
BDL² = a² + a² + a²
(2√2 × r)² = 3a²
8 × r² = 3a²
We will rearrange this equation to solve for the edge length (a):
a = √(8 × r² / 3)
Given that the atomic radius of calcium (r) is 1.97 Å, we can substitute this value into the equation;
a = √(8 × (1.97 Å)²/³)
a = √(8 × 3.8809 Ų / 3)
a = √(30.8072 Ų / 3)
a = √10.2691 Ų
a ≈ 3.206 Å
Therefore, the edge length (a) of the unit cell will be 3.206 Å.
To know more about face-centered cubic lattice here
https://brainly.com/question/29799274
#SPJ4
what mass of sodium phosphate is required to prepare 250.0 ml of a solution that is 0.30 m in sodium ion?
Answers
4.1g of sodium phosphate is required to prepare 250.0 ml of a solution that is 0.30 m in sodium ion.
The important thing to note here is that each mole of trisodium phosphate (\(Na_{3}PO_{4}\)) gives us 3 moles of Na ions. So a solution that is 0.30 M in sodium ion is only 0.10 M in \(Na_{3}PO_{4}\).
Now, molarity is moles/L, so we can figure out the total number of moles we need:
0.10 mol/L × 0.250 L = 0.025 moles \(Na_{3}PO_{4}\).
Finally, the MW of \(Na_{3}PO_{4}\) = 164 g/mol.
So: 0.025 moles × 164 g/mol = 4.1 g
So you would need 4.1 g of trisodium phosphate to make 250 mL of this solution.
Learn more about trisodium phosphate:
brainly.com/question/23286919
#SPJ4
.In the titration of a weak monoprotic acid with a strong base, the pH of the titration solution halfway to the endpoint will be:A. greater than 7.00.B. equal to the pKa of the weak acid.C. greater than the pKa of the weak acid.D. less than the pKa of the weak acid.E. equal to 7.00.
Answers
In the titration of a weak monoprotic acid with a strong base, the pH of the titration solution halfway to the endpoint will be:
C. greater than the pKa of the weak acid.
During the titration of a weak acid with a strong base, the pH of the solution changes as the titrant is added. At the halfway point to the endpoint, the moles of strong base added are approximately equal to half the moles needed to reach the equivalence point.
Since the weak acid is partially neutralized, the concentration of its conjugate base increases, making the solution more basic. As a result, the pH at the midpoint of the titration will be greater than the pKa of the weak acid.
Learn more about the titration: https://brainly.com/question/31870069
#SPJ11
cual es la longitud de una taza con aceite???? AYUDA ES URGENTE!!!
Answers
Answer:
1 taza = 250 ml eso creo
Explanation: Eso creo jejejejeje.
newton's 3rd law: for every_____there is an_____and_____reaction
Answers
There are total three laws of newtons, first law of newtons, second law of newton and third law of newton. Therefore, for every action there is an equal and opposite reaction.
What is newton's third law?Newton's first law is also called law of inertia. An object at rest remains at rest, and an object in motion remains in motion at constant speed and in a straight line unless acted on by an unbalanced force.
Third law of newton states that for every action there is an equal and opposite reaction.
Therefore, for every action there is an equal and opposite reaction.
To know more about newton's law, here:
https://brainly.com/question/29768600
#SPJ1
Anaplasia is recognized by loss of organization and a marked increase in nuclear size. true or false
Answers
The statement "Anaplasia is recognized by loss of organization and a marked increase in nuclear size" is true.
Anaplasia refers to a condition in which cells lose their normal structural and functional characteristics, often associated with malignancy or cancer. This process results in cells becoming less differentiated, losing their organization, and often showing an increase in nuclear size.
In normal cells, differentiation occurs as cells specialize to perform specific functions. However, when anaplasia occurs, cells revert to a less specialized state, which can lead to uncontrolled growth and division. This uncontrolled growth, in turn, contributes to the formation of tumors and can promote the spread of cancerous cells throughout the body.
Anaplastic cells display several distinct features, including large, irregularly shaped nuclei, increased nuclear-to-cytoplasmic ratio, and a higher rate of cell division. These characteristics make anaplastic cells more aggressive and difficult to treat, as they are often more resistant to standard cancer therapies.
In conclusion, anaplasia is a key indicator of malignancy and is characterized by the loss of organization and an increase in nuclear size. Understanding this process can help in the development of new diagnostic tools and targeted therapies for cancer treatment.
To learn more about functional click here
brainly.com/question/30404138
#SPJ11
Anaplasia is recognized by loss of organization and a marked increase in nuclear size - True.
Anaplasia is a disease, frequently linked to malignancy or cancer, in which cells lose their typical morphological and functional properties. As a result of this process, cells lose their organisation, become less differentiated, and frequently exhibit a rise in nuclear size.
Differentiation takes place in healthy cells as they specialise to carry out particular tasks. Anaplasia, on the other hand, causes cells to return to a less specialised condition, which can cause uncontrolled growth and division. In turn, this unchecked proliferation aids in the development of tumours and has the potential to encourage the spread of malignant cells throughout the body.
The nuclear-to-cytoplasmic ratio is elevated, the nuclear size is enlarged, and the rate of cell division is raised in anaplastic cells. Anaplastic cells are more aggressive and challenging to treat due to these traits, as they are frequently more resistant to conventional cancer therapy.
In conclusion, anaplasia, which is characterised by the loss of organisation and an increase in nuclear size, is a significant sign of malignancy. Understanding this procedure can aid in the creation of novel diagnostic techniques and focused cancer therapy.
Learn more about Anaplasia:
https://brainly.com/question/28244480
SPJ4
What pairs of aqueous solutions form percitipate when mixed?
Answers
When silver nitrate and sodium chloride are combined with water, silver chloride will solidify and precipitate out of solution. In this instance, silver chloride is the precipitate.
Which four liquid precipitation examples are there?Precipitation includes the following: rain, hail, sleet, and snow. Rain forms when water vapour in clouds condenses on dust particles, which eventually grow too big to stay in the cloud and fall to the ground, where they collect more water and enlarge further.
What does the precipitation reaction in aqueous solution look like as an example?The chemical reaction between potassium chloride and silver nitrate, in which solid silver chloride precipitated out, is among the greatest examples of precipitation reactions. This precipitation reaction resulted in the formation of an insoluble salt.
To know more about precipitate visit:-
https://brainly.com/question/11387485
#SPJ9
Why did J.J. Thomson reason that electrons must be a pare of the atoms of all elements?
A) Cathode rays are negatively charged particles
B) Cathode rays can be deflected by magnets
C) An electron is 2000 times lighter than a hydrogen atom
D) Charge-to-mass ratio of electrons was the same, regardless of the gas used
Answers
Answer:
a
Explanation:
Why is there such a large jump and ionization energy between the second and third ionization energy‘s for magnesium?