A 1 liter solution contains 0.247 M nitrous acid and 0.329 M sodium nitrite. Addition of 0.271 moles of calcium hydroxide will: (Assume that the volume does not change upon the addition of calcium hydroxide.)
a. Raise the pH slightly
b. Lower the pH slightly
c. Raise the pH by several units
d. Lower the pH by several units
e. Not change the pH
f. Exceed the buffer capacity
Answers
Answer:
a. Raise the pH slightly
Explanation:
We know that
Pka of HNO2/KNO2 =3.39
Moles of HNO2 in the buffer=0.247 mol/L×1L=0.247 moles
Moles of NO2-=0.329mol/L×1L=0.329 moles
If 0.271 moles of Ca(OH)2 is added it will neutralise 0.136 moles of acid ,HNO2,remaining HNO2=0.247-0.136=0.111 moles
Moles of NO2- will increase as 0.0333 moles Ca(NO)2 will be formed =0.0333+0.036=0.0693 moles
pH=pka+log [base]/[acid] {henderson -hasselbach equation}
=3.39+log (0.0693/0.0317)=3.39+0.34=3.73
pH=3.73
Related Questions
How are changes of state different from chemical change?
Answers
Question:
How are changes of state different from chemical change?
Answer:
Physical changes alter only the size, shape, form or matter state of a material. Water boiling, melting ice, tearing paper, freezing water and crushing a can are all examples of physical changes.
On the other hand, chemical changes are a bit different. In a chemical change, a new substance is formed.
Answer:
Chemical change happens when a substance changes they molecular structure, it often involves change in color (rusting iron), transfer of energy (copper smelting), or gas bubbling.
Changes of state is a physical change. An example is ice, it melts to create water. Water then can be freezed back to ice. Water is still H2O in both states(solid/liquid) Dissovling is another example. You can dissolve salt in water and can separate it by evaporating water and leaving the salt behind.
Explanation:
Match the definition with the correct vocab word. - Numbers to Names
Atom
Nucleus
Electron
Neutron
Proton
Matter
1.
Anything that has mass and takes up space.
2.
The basic unit of an element. All matter is made up of atoms.
3.
A positively charged subatomic particle located at the center of an atom.
4.
A subatomic particle with no charge located at the center of an atom.
5.
A subatomic particle of an atom that is negatively charged and orbits the nucleus extremely fast.
6.
Protons and neutrons clump together at the center of an atom to form the nucleus of an atom.
Answers
Answer:
1. Matter
2. Atom
3. Proton
4. Nucleus
5. Electron
6.Neutron
Help pls i really need help

Answers
Answer: I think it's 1
Explanation: Potential energy means stored energy . In Position 1 it's not moving the energy is being stored. Hope that helps.
Write a short essay about life in the Han Dynasty, comparing it to life today. Make sure to include key features:
-Family
-Government
-Social Structure
-Religion
-Trade
Answers
Answer:
Life in the Han Dynasty (206 BCE - 220 CE) differed significantly from today in family, government, social structure, religion, and trade. For example, the Han Dynasty emphasized a patriarchal family structure, where the eldest male held authority, and filial piety was highly valued. In contrast, contemporary societies embrace more egalitarian family dynamics with shared decision-making.
The government system of the Han Dynasty relied on a centralized bureaucracy and emphasized meritocracy, while modern societies often adopted democratic systems. Socially, the Han Dynasty followed a hierarchical model influenced by Confucian principles, whereas contemporary societies strive for greater equality and social mobility.
Religion in the Han Dynasty combined Confucianism, Taoism, and Buddhism, whereas modern societies exhibit diverse religious beliefs. Lastly, trade in the Han Dynasty thrived along the Silk Road, while modern trade was globally interconnected and facilitated by technological advancements. These differences highlight the evolution of society over time.
Explanation:
A 3.69 g
sample of a compound consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur was combusted in excess oxygen. This produced 2.08 g
CO2
and 1.28 g
H2O
. A second sample of this compound with a mass of 4.65 g
produced 4.77 g
SO3
. A third sample of this compound with a mass of 8.62 g
produced 3.48 g
HNO3
. Determine the empirical formula of the compound. Enter the correct subscripts on the given chemical formula.
Answers
The empirical formula of the compound is C₂H₁₆S₂N₃O.
What is the empirical formula of the compound?The moles of each element is as follows::
For CO₂:
Carbon (C) has a molar mass of 12.01 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of C in CO₂ = 2.08 g / 12.01 g/mol = 0.173 moles
Moles of O in CO₂ = 2.08 g / 16.00 g/mol = 0.130 moles
For H₂O:
Hydrogen (H) has a molar mass of 1.01 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of H in H₂O = 1.28 g / 1.01 g/mol = 1.27 moles
Moles of O in H₂O = 1.28 g / 16.00 g/mol = 0.080 moles
For SO₃:
Sulfur (S) has a molar mass of 32.06 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of S in SO₃ = 4.77 g / 32.06 g/mol = 0.149 moles
Moles of O in SO₃ = 4.77 g / 16.00 g/mol = 0.298 moles
For HNO₃:
Hydrogen (H) has a molar mass of 1.01 g/mol.
Nitrogen (N) has a molar mass of 14.01 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of H in HNO₃ = 3.48 g / 1.01 g/mol = 3.45 moles
Moles of N in HNO₃ = 3.48 g / 14.01 g/mol = 0.248 moles
Moles of O in HNO₃ = 3.48 g / 16.00 g/mol = 0.217 moles
The simplest whole-number ratio of the elements will be:
Carbon: 0.173 moles / 0.080 moles ≈ 2.16
Hydrogen: 1.27 moles / 0.080 moles ≈ 15.88
Sulfur: 0.149 moles / 0.080 moles ≈ 1.86
Nitrogen: 0.248 moles / 0.080 moles ≈ 3.10
Oxygen: 0.080 moles / 0.080 moles = 1
Therefore, the empirical formula is C₂H₁₆S₂N₃O.
Learn more about empirical formulas at: https://brainly.com/question/1603500
#SPJ1
It takes 12.35 grams of acetic acid (CH3COOH; molar mass of 60.05 g/mol) to prepare a
1.5 M solution. What is the volume in L?
Answers
Answer:
\(V=0.137L\)
Explanation:
Hello.
In this case, since the molarity is computed in terms of the moles of solute (acetic acid) and the volume of the solution in liters, we must first compute the moles of acetic acid by using its molar mass to subsequently compute the volume as follows:
\(n=12.35g*\frac{1mol}{60.05g}=0.2057mol\)
\(M=\frac{n}{V}\\ \\V=\frac{n}{V}=\frac{0.2057mol}{1.5mol/L}\\ \\V=0.137L\)
Best regards.
The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2
in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
Answers
d.2.93m.CaCl2 is present in the solution with a 2.93m concentration.
The boiling point of a solution is directly related to its concentration. The boiling point elevation of a solution, ΔTb, is equal to the product of the van't Hoff factor (i) and the molality of the solution (m).The quantity of moles of solute per kilogramme of solvent is known as molality.
Therefore, we can solve for the molality of the solution using the following equation:
ΔTb
\(= i *m\\105.3\°C= i * m\\\)
\(m =\frac{ 105.3 \°C }{i}\)
Assuming an ideal van't Hoff factor for CaCl2 (i = 2), the molality of the solution is:
\(m =\frac{ 105.3 \°C }{ 2}\\m = 52.65 m = 52.65 mol/kg\)
The concentration of CaCl2 in the solution is then:
\(C = m * Kb\\C = 52.65 mol/kg * 0.512 \°C/m\\C = 2.93 mol/kg\)
Therefore,The concentration of CaCl2 in the solution is 2.93m.
learn more about boiling point refer:brainly.com/question/24168079
#SPJ1
complete question:The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2 in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
a.3.45m
b.4.40m
c.8.79m
d.2.93m
what is the correct equation for water gas
Answers
Answer:
Water-gas A mixture of carbon monoxide (CO) and hydrogen (H2) produced by passing steam over red-hot coke using the endothermic reaction C + H2O # CO + H2.
Explanation:
Find the grams in 6.25 moles of HC2H302.
Answers
use this formula m=n×M
Your n would be 6.25mols
Your Molar mass would be the number you get after adding the molar mass of your equation: HC2H302. Find your periodic table and look there.
And you plug in the molar number in the formula and you multiply n×M
I would help but I don't have the periodic table with me right now
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
If a 60-newton force pushes a cart to the right, and a 20-newton force pushes the cart to the left, what is the net force on the cart? What direction is it in?
Answers
Answer:
I think it's 40 and to the right?
Explanation:
60-20 equals 40, and the force to the right is greater so it's to the right.
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers
Answer:
Volume of the gass will decrease by three times of the original volume
Explanation:
Volume is inversly propotional to the pressure applied on it.
Answer:
it is decreased to one third of its original volume
Explanation:
how would you prepared pure water from the mixture of impure water? explain with experiment.
Answers
Explanation:
Hello, there are alot method to separate the impurities from water. One of those & best method for purification of water is Distillation.
During distillation the given apparatus are set as shown in figure. After arrangement we put the impure water in distillation flask & there are two mouth of flask one is attached to condenser & other is covered. There are two tubes in the middle of the condenser in which water comes in from one and water goes out from the other and this happens continuously. The condenser is used to convert the collected water vapours into liquid water so that it can be collected easily in recieving flask.
\( \small \sf Thanks \: for \: joining \: brainly \: community! \)

What is the oxidation state of N in NaNOz?

Answers
The oxidation state of nitrogen (N) in NaNO3 is +5. option B
To determine the oxidation state of nitrogen (N) in sodium nitrate (NaNO3), we need to assign oxidation numbers to each element in the compound.
In NaNO3, we know that the sodium ion (Na+) has a +1 oxidation state because it is an alkali metal. Oxygen (O) typically has an oxidation state of -2 in compounds, and there are three oxygen atoms in NaNO3. Since the compound is neutral, the sum of the oxidation states must be zero.
Let's assume that the oxidation state of nitrogen is x. Therefore, we can set up the equation:
(+1) + x + (-2) * 3 = 0
Simplifying the equation:
+1 + x - 6 = 0
x - 5 = 0
x = +5
Therefore, the oxidation state of nitrogen (N) in NaNO3 is +5.
The oxidation state of an element indicates the number of electrons it has gained or lost in a compound. In this case, the nitrogen atom in NaNO3 has gained five electrons to achieve a stable oxidation state of +5.
It is important to note that oxidation states are formal charges and do not necessarily represent the actual distribution of electrons in a compound. They are assigned based on a set of rules and can be useful in understanding the reactivity and behavior of elements in chemical reactions.
Option B
For more such questions on oxidation state visit:
https://brainly.com/question/25551544
#SPJ8
Who created the periodic table?
Answers
Answer:
Dmitri Mendeleev=Russian chemist, Albert Ghiorso=American scientist
Explanation:
hexaphosphorus nonasulfide formula
Answers
Answer:
P6S9
Explanation:
Firstly, let's write the numbers in Latin
1 = mono
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
Secondly, write the symboles of the given elements:
Phosphorus is P
Sulfide is S
Finally, connect the numbers and symbols.
Rule of pronunciation: Number of first element + symbol of first element + number of second element + symbol of second element
P6S9
Please upvote.
A box measures 8.54 ft in length, 0.0455 yd in width and 9.18 inches in height. What is its volume in cubic centimeters (cm3)?
1 yard = 3 ft 1 in = 2.54 cm 1 ft = 12 in
HINT: Break each given number down SEPARATELY. So 3 different dimensional analysis converting each number to centimeters. THEN take those 3 converted numbers for length, width and height and plug them into the volume equation. Volume = length x width x height. If you think about the units of centimeters being multiplied by each other (cm x cm x cm) in the volume equation, they all combine to form cubic centimeters (cm3).
Answers
The volume in cubic centimeters is 25251.17cm³
Volume measures capacity. So the unit of volume is basically a unit for measuring the capacity or the extent of an object or space. The unit is mostly used to specify the volume of goods or a liquid (fluids).
In this problem, volume of the box is given by the formula:
V = l x w x h
Solving the problem
First, let us convert every dimension to centimeter.
l = 8.54 ft x 12 inches x 2.54 cm = 260.29 cm
w = 0.0455 yd x 3ft x 12 inch x 2.54 cm = 4.16052cm
h = 9.18 in x 2.54 cm = 23.3172 cm
Now, we can solve for the volume of the box using the given formula above.
V = l x w x h
Then substitute the given data
V = (260.29 cm) x (4.16052 cm) x (23.3172 cm)
V = 25251.17cm³
Learn more about Volume units, here:
https://brainly.com/question/12347336
#SPJ1
Which of these gaseous elements is LEAST reactive?
a. hydrogen
b.helium
c.nitrogen
d.oxygen
Answers
Answer:
helium
Explanation:
it has 8 valence electrons which fills its outer energy level making it have a stable arrangement of electrons hence least reactive
given the atomic mass of hydrogen is 1 amu, the atomic mass of oxygen is 16 amu, and one molecule of sulfuric acid has a mass of 98 amu, what is the atomic mass of sulfur trioxide?
Answers
The atomic mass of sulfur trioxide (SO3) is 82 amu.
How to find the atomic mass of sulfur trioxide ?Sulfur trioxide (SO3) has one sulfur atom and three oxygen atoms.
The atomic mass of sulfur can be calculated by subtracting the total mass of the oxygen atoms in sulfuric acid (3 x 16 amu) from the mass of sulfuric acid (98 amu) and then subtracting the mass of the remaining oxygen atom:
Mass of sulfur = (98 amu - 3 x 16 amu) - 1 x 16 amuMass of sulfur = (98 amu - 48 amu) - 16 amuMass of sulfur = 34 amuThe atomic mass of sulfur is 34 amu.
To find the atomic mass of sulfur trioxide, we add the atomic masses of one sulfur atom and three oxygen atoms:
Atomic mass of SO3 = 1 x 34 amu + 3 x 16 amuAtomic mass of SO3 = 34 amu + 48 amuAtomic mass of SO3 = 82 amuTherefore, the atomic mass of sulfur trioxide (SO3) is 82 amu.
Learn more about sulfur trioxide here : brainly.com/question/1458186
#SPJ1
Given the following equation: C2H60 + 302 + 3H2O + 2CO2
If 2.0 moles of CO2 were produced, how many moles of O2
reacted?
Answers
Answer:
3moles
Explanation:
relation taken from balanced equation
Calculate the mass of NaCl in a 44 −mL sample of a 1.6 M NaCl solution
Answers
The mass of NaCl in 44mL of a 1.6M solution is 4.1grams.
How to calculate mass?The mass of NaCl can be calculated by using the following formula;
Molarity = no. of moles ÷ volume
Molarity is the concentration of a substance in solution, expressed as the number moles of solute per litre of solution.
no of moles = 1.6M × 0.044L = 0.07moles
The mass of the sodium chloride solution can be calculated by multiplying the molar mass of the salt by the number of moles present in the solution.
The molar mass of NaCl solution can be calculated as follows;
M.M = 23g/mol + 35.5g/mol = 58.5g/mol
mass of NaCl = 58.5 × 0.07 = 4.1grams.
Therefore, 4.1grams is the mass of NaCl solution.
Learn more about molarity at: https://brainly.com/question/12127540
#SPJ1
O2 is which of the following?
Both compound and molecule
One atom
compound
Molecule
Answers
The chemical formula O₂ which is oxygen is a molecule as it is made up of 2 oxygen atoms.
What is chemical formula?
Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
It is not the same as structural formula and does not have any information regarding structure.It does not provide any information regarding structure of molecule as obtained in structural formula.
Learn more about chemical formula,here:
https://brainly.com/question/29031056
#SPJ1
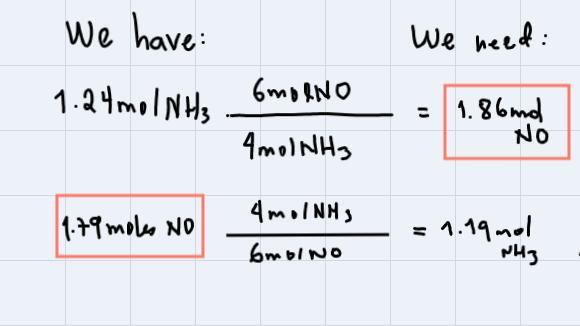
In the reaction below, what is the limiting reactant when 1.24 moles NH of reacts with 1.79 moles of NO?
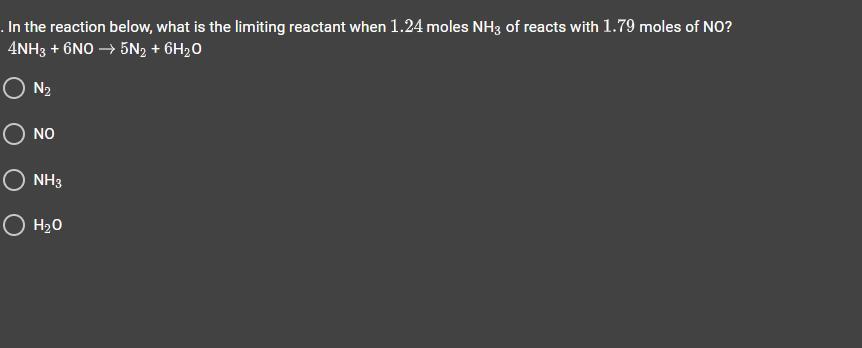
Answers
To identify the limiting reactant, we use the coefficients of the reaction:
As you can see, we have 1.79 moles of NO, but we need 1.86 moles of NO according to the reaction. For this reason, NO is the limiting reactant since we need more than what we have.
combustion always result in to formation of water. what other type of reactions may result into formation of water? examples of these reactions
Answers
As combustion always result into the formation of water, the other type of reactions that may result into formation of water are Acid-Base Neutralization Reactions and Hydrogen and Oxygen Reaction.
Acid-Base Neutralization Reactions:
A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.
H⁺ ions and OH⁻ ions combine to generate water during a neutralisation reaction. Acid-base neutralisation is the most common type of neutralisation reaction.
Example: Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H₂O
Hydrogen and Oxygen Reaction:
Water vapour is created when hydrogen gas (H₂) and oxygen gas (O₂) are combined directly. This reaction produces a lot of heat and releases a lot of energy.
Example: 2 H₂ + O₂ → 2 H₂O
Learn more about reactions:
https://brainly.com/question/25769000
(c) 45 g C,H, react with 45 g Cl₂ according to the equation:
Cl₂ + C6H6 C6H5Cl + HCI. What is the limiting reactant? What mass of HCI will be produced?
-
Answers
In the given reaction, the limiting reactant is C₆H₆ (benzene).
To determine the limiting reactant as well as calculate the mass of HCl produced, compare the moles of each reactant.
The number of moles for each reactant:
Molar mass of Cl₂ = 35.5 g/mol + 35.5 g/mol = 71 g/mol
Moles of Cl₂ = mass of Cl₂ / molar mass of Cl₂
= 45 g / 71 g/mol
= 0.634 moles of Cl₂
Molar mass of C₆H₆ (benzene) = 12 g/mol + 6(1 g/mol) = 78 g/mol
Moles of C₆H₆ = mass of C₆H₆ / molar mass of C₆H₆
= 45 g / 78 g/mol = 0.577 moles of C₆H₆
Determine the stoichiometry between Cl₂ and HCl:
Cl₂ + C₆H₆ → C₆H₅Cl + HCl
Here, we can see that 1 mole of Cl₂ produces 1 mole of HCl.
Thus, the limiting reactant is C₆H₆ (benzene).
Calculate the mass of HCl produced:
Molar mass of HCl = 1 g/mol + 35.5 g/mol = 36.5 g/mol
Moles of HCl produced = moles of C₆H₆ = 0.577 moles
Mass of HCl produced = moles of HCl produced × molar mass of HCl
Mass of HCl produced = 0.577 moles × 36.5 g/mol
≈ 21.04 g
Therefore, approximately 21.04 grams of HCl will be produced.
For more details regarding limiting reactant, visit:
https://brainly.com/question/10090573
#SPJ1
Given the reaction at equilibrium:
2NO2(g) → N204(g) Heat of
reaction is -55.3 kJ) What type of
reaction is this?
O Endothermic
O Exothermic
Answers
When the equilibrium constant is higher than one, it indicates that the reaction prefers to produce products, whereas if the equilibrium constant is less than one, it indicates that the reaction prefers to produce reactants. If the equilibrium constant is equal to one, the reaction proceeds in both directions equally.
In a chemical reaction, exothermic reactions are defined as reactions that release heat into their environment. It implies that heat is given off when reactants are converted to products. At equilibrium, an exothermic reaction continues to be exothermic, meaning that heat is given off even after the reaction reaches a state of equilibrium.There are two types of reactions: exothermic and endothermic.
A reaction is classified as exothermic if it releases heat, and endothermic if it absorbs heat. The direction of the reaction is determined by whether it is exothermic or endothermic. At equilibrium, the reaction is no longer moving forwards or backwards. It's also worth noting that reactions can be exothermic in one direction and endothermic in the other.
The equilibrium constant (K) is defined as the ratio of the concentration of products to the concentration of reactants in the chemical reaction equation. It is used to express how much of the products is generated by the reaction in comparison to the reactants. the equilibrium constant aids in the identification of the direction in which the reaction will proceed at equilibrium.
for more questions on equilibrium
https://brainly.com/question/3159758
#SPJ8
Which statement best illustrates how mixtures and pure substances are different?
Mixtures have color; pure substances are colorless.
Mixtures have various odors; pure substances are odorless.
Mixtures are found on the periodic table; pure substances are not.
Mixtures are physically combined; pure substances are chemically combined.
Answers
Answer:
Mixtures are physically combined and pure substances are chemically combined.pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
Please show me how you do and please involve the units

Answers
The question requires us to calculate the amount of energy absorbed by the reaction, considering the molar enthalpy for oxygen gas (O2) and that 80.6 g of oxygen were reacting.
The following information was provided by the question:
Balanced chemical reaction:
\(N_{2(g)}+2O_{2(g)}\to N_2O_{4(g)}\)Molar enthalpy of reaction for O2: +499.0 kJ/mol
Mass of O2 reacting: 80.6 g
To solve this problem, we need to calculate the amount of moles that corresponds to the mass of O2 given and then use this value and the molar enthalpy provided to calculate how much heat would be absorbed by the reaction.
First, we need the molar mass of O2. Knowing that the atomic mass of O is 15.99 u:
molar mass (O2) = (2 * 15.99) = 31.98 g/mol
Now, we use the molar mass to calculate the number of moles in 80.6 g of O2:
31.98 g O2 ------------------------ 1 mol O2
80.6 g O2 ------------------------- x
Solving for x, we'll have:
\(x=\frac{(80.6\text{ g O}_2)\times(1\text{ mol O}_2)}{(31.98\text{ g O}_2)}=2.52\text{ mol of O}_2\)There are 2.52 moles of O2 reacting and next we need to calculate the amount of heat absorbed considering this.
The molar enthalpy of O2 tells us how much heat is abosrbed when 1 mol of O2 reacts. Thus, we can use it to calculate the amount of heat absorbed when 2.52 moles of O2 react:
1 mol O2 ----------------------- 499.0 kJ
2.52 mol O2 ----------------- y
Solving for y, we'll have:
\(y=\frac{(2.52\text{ mol O}_2)\times(499.0\text{ kJ)}}{(1\text{ mol O}_2)}=1257.5\text{ kJ}\)Therefore, 1257.5 kJ of energy are absorbed when 80.6 g of O2 are reacting.
We can fill in the boxes to answer the question as it follows:
Answer: 1257.5
Units: kJ
What is the relationship between radius and diameter
Answers
Answer:
the radius is half the diameter
The radius of a circle is equal to half the diameter.