58:27
1
Dominic made the table below to organize his notes about mixtures
Properties of Mixtures
has no set composition
must have more than one state of matter
must have more than one substance
What mistake did Dominic make?
The title should read "Properties of Solutions" because some mixtures do not have all of the properties listed
O There is a definite recipe to make each mixture, so the composition of a mixture is set.
Although it is possible to have more than one state, it is also possible to have only one state.
O A single substance can be used to make a mixture if the substance is composed of more than one element
Sao and Fit
Answers
Answer:
Although it is possible to have more than one state, it is also possible to have only one state.
Explanation:
The mistake Dominic made was stating that "although it is possible to have more than one state, it is also possible to have only one state."
Mixtures are impure substances with the following properties:
They have an indefinite composition
Their constituents retains their identities.
Constituents react differently to changed conditions.
They can easily be separated into constituents by physical methods.
There are two types of mixtures based on the number of phases coexisting:
Homogeneous mixtures have their constituents existing in just one phase.
Heterogeneous mixtures exists in at least two different phases.
Explanation:
Related Questions
How many milliliters of 8.54×10−2 M Ba(OH)2(aq) are required to titrate 54.90 mL of 5.14×10−2 M HNO3
Answers
16.52 milliliters of 8.54×10⁻² M Ba(OH)2(aq) are required to titrate 54.90 mL of 5.14×10⁻² M HNO₃
What is titration?A titration is a procedure that uses a known solution to determine the concentration of an unknown solution. Until the reaction is finished, the titrant (the known solution) is typically supplied from a buret to a known volume of the analyte (the unknown solution).
Titration is an essential technique in analytical chemistry, and it is also known as volumetric analysis.
n-factor for Ba(OH)₂ =2
Thus, dilution equation becomes:
n × M₁V₁= M₂V₂
2 x 8.54 x 10⁻² x V₁ = 5.14 x 10⁻² x (54.90/1000)
V₁= 16.52 × 10⁻³¹
V₁ = 16.52 ml
Thus, 16.52 milliliters of 8.54×10⁻² M Ba(OH)2(aq) are required to titrate 54.90 mL of 5.14×10⁻² M HNO₃
To know more about titration refer to:
https://brainly.com/question/13307013
#SPJ1
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
Which part of the manufacturing process occurs when binders are added to a paint mixture?
Answers
Answer:
I know this was 2 weeks ago, but it was combining.
Explanation:
Answer:
Combining
Explanation
Part C
Convert 8x10-4 kg to micrograms.
Express your answer with the appropriate units. Use the appropriate metric symbols for the units.
VO 5 ΑΣΦ
Submit
→
Request Answer
?
ug
Answers
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
The table compares the type of reactants and products taking part in two chemical changes.
Which of the following statements correctly identifies the synthesis reaction and explains why?
Only chemical reaction X, because one reactant is a compound
Only chemical reaction Y, because both reactants are elements
Neither chemical reaction X nor Y, because they have only one product
Both chemical reactions X and Y, because there are two reactants and a single product
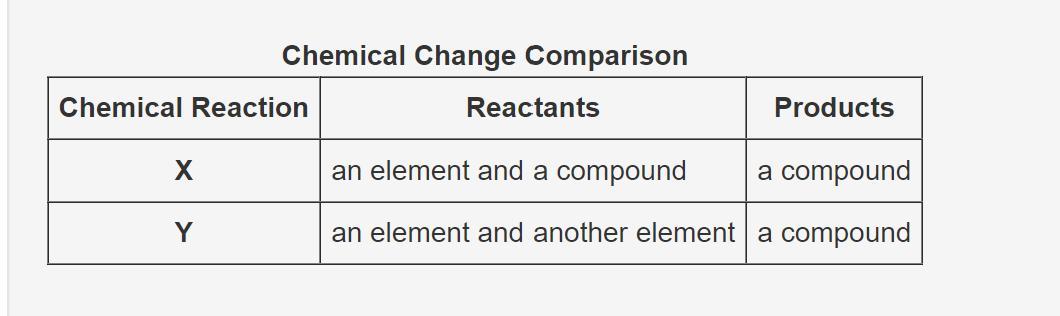
Answers
Both chemical reactions X and Y are possible because there are two reactants and one product in synthesis reaction
What is true about synthesis reaction?
When two or more reactants are present, a synthesis reaction produces a single product that is more complex than the initial reactants.
This kind of reaction is described by the general equation A + B = AB. Synthesis reactions are those in which two reactants combine to form a chemical. Synthesis reactions involve the chemical mixing of two or more substances to create a single compound or end product.
To know more about synthesis reaction characteristics, visit :
brainly.com/question/26362040
#SPJ1
How many significant figures is this and 40.5184 rounded
Answers
Answer:
forty point five one eight four
Explanation:
40.5184
Sig Figs
6
40.5184
Decimals
4
40.5184
Scientific Notation
4.05184 × 101
E-Notation
4.05184e+1
Words
forty point five one eight four
—————
—————
—————
\/

What types of orbitals are found in the third energy level?
Answers
Answer:
1 s-orbital, 3 p-orbital and 5 d-orbital
Answer:
there are a total of nine orbitals in a third energy level
Explanation:
How many decigrams are equal to 1.35 milligrams? 135 dg 13.5 dg 0.0135 dg 0.135 dg
Answers
Answer:
0.0135 dg
Explanation:
Answer: 0.0135 dg
Explanation:
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Which of the following objects would have the smallest wavelength at the same velocity?
Baseball
Electron
Planet Earth
Proton
Answers
Answer: Planet Earth
Explanation: Wavelength (λ) is equal to h/mυ , where m = the mass of your object and υ = the frequency. Since Earth has the largest mass of the options, we can conclude that Earth will have the smallest wavelengths out of the rest.
The wavelength of matter-waves is given by the De Broglie formula. The larger the object, the smaller the observed wavelength hence the object having the smallest wavelength is Planet Earth.
De Broglie established the relationship between the momentum and the observed wavelength of an object. This proposition came to be known as wave-particle duality.
The wavelength of matter waves is given by;
λ = h/mv
Where;
λ = wavelength
m = mass of object
v = velocity of the object
If all the objects have the same velocity, the magnitude of the wavelength now depends on the mass of the object.
The object having the largest mass among the options is planet earth (5.972 × 10^24 kg).
Hence planet earth has the smallest wavelength among the options listed.
Learn more; https://brainly.com/question/7143261
CH3CH2CH(OH)CH3 enantiomers using 3D structure
Answers
The enantiomers of CH3CH2CH(OH)CH3 are (R)-2-butanol and (S)-2-butanol and non-superimposable mirror images of each other.
What are the CH3CH2CH(OH)CH3 enantiomers?CH3CH2CH(OH)CH3, also known as 2-butanol, has one chiral center, the carbon atom bonded to the hydroxyl group (OH) and three other groups (methyl, ethyl, and hydrogen).
Therefore, it can exist in two enantiomeric forms, (R)-2-butanol and (S)-2-butanol. The enantiomers are non-superimposable mirror images of each other and have different optical activities.
Learn more about enantiomers at: https://brainly.com/question/30216513
#SPJ1

Given: H2 + O 2 → H2O1
the reaction occurs at ST.P a) Balance the chemical equation. (1 pts) b) Calculate the number of moles of the reactants needed to obtain 45 liner of H2O (2 pt) 4) Deduce the volume of the reactants (2 pts)
Answers
a) The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) the number of moles of O₂ required is approximately 1.004 moles.
c) approximately 45 liters of H₂ and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O.
a) Balancing the chemical equation:
The balanced chemical equation for the reaction is: 2H₂ + O₂ → 2H₂O
b) Calculating the number of moles of the reactants needed to obtain 45 liters of H₂O:
From the balanced equation, we can see that for every 2 moles of H₂O produced, we need 2 moles of H₂ and 1 mole of O₂. Since the stoichiometry is based on moles, we need to convert the given volume of H2O into moles.
To convert volume to moles, we need to use the ideal gas law, PV = nRT. At standard temperature and pressure (STP), the molar volume of an ideal gas is 22.4 liters.
Given that we have 45 liters of H2O, we can calculate the number of moles as follows:
moles of H₂O = (volume of H₂O) / (molar volume at STP)
= 45 liters / 22.4 liters/mol
≈ 2.008 moles of H₂O
Since the stoichiometry of the reaction is 2 moles of H₂O for every 2 moles of H₂, we need an equal number of moles of H₂. Therefore, the number of moles of H₂ required is also approximately 2.008 moles.
For O₂, since the stoichiometry is 1 mole of O₂ for every 2 moles of H₂O, we need half the number of moles of H₂O. Thus, the number of moles of O₂required is approximately 1.004 moles.
c) the volume of the reactants:
Since the stoichiometry of the balanced equation is 2 moles of H₂for every 1 mole of O₂ and 2 moles of H₂O, we can deduce the volume of the reactants based on their molar volumes at STP.
For 2.008 moles of H₂, the volume can be calculated as follows:
volume of H₂= (moles of H₂) * (molar volume at STP)
= 2.008 moles * 22.4 liters/mol
≈ 45 liters of H₂
For 1.004 moles of O₂, the volume can be calculated similarly:
volume of O₂= (moles of O₂) * (molar volume at STP)
= 1.004 moles * 22.4 liters/mol
≈ 22.5 liters of O₂
Therefore, approximately 45 liters of H₂and 22.5 liters of O₂ are needed to obtain 45 liters of H₂O
for more questions on chemical
https://brainly.com/question/29886197
#SPJ8
Given the law of conservation of energy, what happens when a 200°C iron bar is placed in thermal contact with a 30°C block of wood?
Answers
When a 200°C iron bar is placed in thermal contact with a 30°C block of wood, energy leaves the iron bar and enters the wood until the temperatures are equal.
Law of conservation of energy states that the energy cannot be lost or formed but it can only be transformed from one form to another.
According to the given question, the block of wood is at a lower temperature than an iron bar. Hence, heat will flow from the iron bar to the block of wood until the temperatures of both are equal.
Know more about the Law of conservation of energy,
https://brainly.com/question/24772394
https://brainly.com/question/11549071
11 . A guest orders a drink that contains 4 1/2 ounces of 80-proof liquor. Approximately how many drinks does this beverage contain?
Answers
From the calculations that have been done, there are 3 drinks.
How many drinks are there?The information that we have been provided here is very important. We can obtain the amount of alcohol by looking at the fact that it contains 80-proof liquor.
This 80-proof liquor. is an equivalent of 40% alcohol this means that there is 1.5 ounce of distilled spirit.
Thus, the number of drinks there is;
4.5/1.5 = 3
Learn more about alcohol:https://brainly.com/question/24255571
#SPJ1
What is the density of an object with a volume of 3ml and a mass of 18 grams
Answers
Answer:
The answer is 6 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\ \)
From the question we have
\(density = \frac{18}{3} \\ \)
We have the final answer as
6 g/mLHope this helps you
Which formula represents a nonpolar molecule containing polar covalent bonds?
Answers
Answer:
Figure 2 represents a nonpolar molecule containing polar covalent bonds
Explanation:
The CO2 molecule is linear, formed by 3 atoms and the electronegativity variations are equal, so its dipolar moment will be null, and this molecule will be nonpolar, however, it has polar covalent bonds.
A student sets up the following equation to convert a measurement fill in the missing part of this equation(0.050 ug/dL)• [] = ? g/mL
Answers
Answer:
To convert a measurement of 0.050 ug/dL to g/mL, we need to multiply it by a conversion factor that relates micrograms (ug) to grams (g) and deciliters (dL) to milliliters (mL).
1 ug = 0.000001 g (or 1 g = 1,000,000 ug)
1 dL = 100 mL
So, the missing part of the equation would be:
(0.050 ug/dL) • (0.000001 g/ug) • (1 dL/100 mL) = 0.0000000005 g/mL
Therefore, the answer is 0.0000000005 g/mL.
Explanation:
Please help need ASAP!!! Due tonight,
Name the Compounds. Spelling counts
SiCl4
N2O4

Answers
Answer:
Silicon tetrachloride
Dinitrogen tetroxide
The name of the compound SiCl₄ is named silicon tetrachloride and N₂O₄ is named dinitrogen tetroxide.
SiCl₄ is named silicon tetrachloride. It is composed of one silicon atom (Si) bonded to four chlorine atoms (Cl). The prefix "tetra-" indicates the presence of four chlorine atoms bonded to the central silicon atom.
N₂O₄ is named dinitrogen tetroxide. It consists of two nitrogen atoms (N) bonded to four oxygen atoms (O). The prefix "di-" indicates the presence of two nitrogen atoms, and the suffix "-tetraoxide" indicates the presence of four oxygen atoms.
Learn more about compound here:
https://brainly.com/question/14117795
#SPJ 6
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Did the candle continue it's flame when covered with a jar for a few minutes?why?
Answers
Answer:
yes it will because the air in the jar is co2 or o2 and these gases have the capacity to burn things out
Which change to the ecosystem had the largest effect on the the population of trout in Wisconsin?
Answers
The largest effect on the population of trout in Wisconsin was caused by the introduction of non-native species into the ecosystem.
The introduction of non-native species into an ecosystem can cause a disruption in the food chain, leading to a decrease in the population of native species. In Wisconsin, the introduction of non-native species such as the brown trout and rainbow trout has had a significant impact on the population of native brook trout.
The non-native species compete with the native brook trout for food and habitat, which has led to a decrease in the brook trout population. This highlights the importance of preserving the natural balance of ecosystems and avoiding the introduction of non-native species.
To know more about ecosystem, click here.
https://brainly.com/question/13979184
#SPJ1
2. Acceleration is______
proportional to mass,
Answers
Answer:
Inversely
Explanation:
The acceleration of an object as produced by a net force is directly proportional to the magnitude of the net force, in the same direction as the net force, and inversely proportional to the mass of the object.
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
How does increasing the pressure affect the reaction rate? A. The activation energy of the reaction is changed. B. The concentration of reactants is changed. C. The temperature of the reaction is changed. D. The phase the reactants are in is changed
Answers
Answer:
Explanation:
b
The rate of the reaction determines the product formation and depends on various factors. The increase in pressure will change the concentration of reactants. Thus, option B is correct.
What is the reaction rate?The rate of the reaction is the speed at which the chemical reaction moves in a direction to yield products. The reaction rate is influenced by the surface area of the substance, temperature, and concentration.
The pressure directly affects the concentration which in turn affects the reaction rate. The increased pressure raises the concentration and more collisions will occur between the particles resulting in a fast rate of reaction.
Therefore, the increase in pressure increases the concentration and reaction rate.
Learn more about reaction rate here:
https://brainly.com/question/12866245
#SPJ5
convert 8.42x10^8 mol/(kg*m^2) to mol/(g*cm^2)
Answers
Answer:
gguhg
Explanation:
no te es caso drama me están muy una las y y que las te
1 kg = 1000 g
1 m = 100 cm
Using these equations, 8.42x10^8 mol/(kgm^2) can be converted to 8.42x10^11 mol/(gcm^2).
Do Metals or Nonmetals have larger ion size compared to the size of the neutral atom?
A. Metals
B. Nonmetals
PLZ HELP ITS 50 POINTS DONT JUST PUT A RANDOM ANSWER OR I WILL REPORT
Answers
Answer:
b
Explanation:
Answer:B. nonmetals
Explanation:
The mass of sample X is 20.0g. It was placed in a graduated cylinder and the water level rose from A to B. What is the density of sample x
Answers
Answer:
4
Explanation:
D=M/V
D=20.0/5
D=4
what is Quantum mechanics??
Can some one summarize this?
Answers
Answer:
its a theory
Explanation:
dealing with the behaviour of matter and light on the atomic and subatomic scale.