58 grams of which substance can be dissolved in 100 grams of water at 50 Celsius?
Answers
The amount of a substance that can be dissolved in 100 grams of water at 50 Celsius depends on the solubility of the substance.
Solubility is the ability of a substance to dissolve in a solvent, such as water, to form a homogeneous solution. The solubility of a substance is affected by temperature, pressure, and the nature of the solute and solvent.
Without knowing the specific substance in question, it is difficult to accurately determine how much can be dissolved in 100 grams of water at 50 Celsius. However, generally speaking, the solubility of most substances increases with increasing temperature. This means that more of the substance can be dissolved at higher temperatures than at lower temperatures.
In conclusion, the amount of a substance that can be dissolved in 100 grams of water at 50 Celsius depends on the solubility of the substance, which is affected by temperature, pressure, and the nature of the solute and solvent. Without knowing the specific substance, it is difficult to accurately determine how much can be dissolved.
Learn more about Solutions and Solubility here:https://brainly.com/question/23946616
#SPJ11
Related Questions
4. what is the shorthand electron configuration of a potassium ion (k )?
Answers
The shorthand electron configuration of a potassium ion (k ) is
[Ar] 4s1.
The shorthand electron configuration of a potassium ion (K+) is [Ar] 4s1. This means that the electron configuration is based on the electron configuration of Argon (Ar), which is a noble gas immediately preceding potassium in the periodic table. The electron configuration of Argon is [Ar] 3d10 4s2. To form a potassium ion, one electron is removed from the 4s orbital, leaving [Ar] 4s1. This is the shorthand electron configuration of a potassium ion.
To know more about electronic configuration please refer:
https://brainly.com/question/29757010
#SPJ4
I NEED HELP ASAP!!!!!!!!!!!!!!

Answers
Answer:
A
Explanation:
N2 is nonpolar and has a triple covalent bond
explain completely how/why atomic absorption spectroscopy allows the quantitation of copper in the presence of other metal ions
Answers
Atomic absorption spectroscopy is an analytical technique that is used to determine the amount of a specific element in a sample. It works based on the principle of absorption of light of a particular wavelength by an element in the ground state when it is excited by a high-energy light source like a flame, an electrode or a laser.
As copper absorbs light of a specific wavelength, this technique can be used to quantify the amount of copper in the presence of other metal ions. The specificity of the method lies in the use of monochromatic radiation, which is only absorbed by copper, and the fact that the concentration of copper in the sample can be calculated based on the extent of light absorption by the sample. Typically, the sample is atomized and then exposed to a beam of light of a specific wavelength. As the light passes through the sample, it is absorbed by the atoms of copper present in the sample. The extent of absorption is measured by a detector, and the amount of copper in the sample is calculated using a calibration curve.
The calibration curve is generated by measuring the absorption of a series of standard solutions of known copper concentration. The presence of other metal ions does not interfere with the measurement of copper concentration by atomic absorption spectroscopy. The use of monochromatic radiation ensures that the light is absorbed only by copper, and the concentration of copper is calculated based on the extent of light absorption. Other metal ions in the sample do not absorb light of the specific wavelength used, and therefore do not contribute to the absorption measured by the detector.
To know more about copper refer to:
https://brainly.com/question/26616184
#SPJ11
Use the following chart of boiling point temperatures to answer the following questions: Elemental form H2 He Li(s) Be(s) Ra B(s) cis) N Melting point 13.81 K 0.95 K 453.65 K 1560 K 2348K 3823 K 63.15 K Boiling point 20.28 K 4.22 K 1615 K 2744K 4273 K 4098 K 77.36 K Name hydrogen helium lithium beryllium boron carbon nitrogen O 54.36 K 90.20 K oxygen F Ne 53.53 K 24.56 K 85.03 K 27.07 K fluorine | neon a. List the elemental forms that have the lower boiling points? What type of bonding and/or interactions might be present for each of the elemental forms you listed for lower boiling points? b. List the elemental forms that have the higher boiling points? What type of bonding and/or interactions might be present for each of the elemental forms you listed for higher boiling points?
Answers
a. The elemental forms with lower boiling points are:
- Hydrogen (H2) with a boiling point of 20.28 K
- Helium (He) with a boiling point of 4.22 K
- Nitrogen (N) with a boiling point of 77.36 K
- Oxygen (O) with a boiling point of 90.20 K
- Fluorine (F) with a boiling point of 85.03 K
- Neon (Ne) with a boiling point of 27.07 K
These elements have lower boiling points because they have weak van der Waals forces or London dispersion forces as the main type of interaction between their molecules or atoms.
b. The elemental forms with higher boiling points are:
- Lithium (Li(s)) with a boiling point of 1615 K
- Beryllium (Be(s)) with a boiling point of 2744 K
- Boron (B(s)) with a boiling point of 4273 K
- Carbon (C(s)) with a boiling point of 4098 K
These elements have higher boiling points because they have strong covalent bonds, ionic bonds, or metallic bonds as the main type of interaction between their atoms or ions.
To know more about boiling point refer here:
https://brainly.com/question/14771622?#
#SPJ11
Which of the following is not a fundamental particle
2) Neutron
1) Proton
3) a-particle
4) Electron
Answers
3)a-particle.
What is the Sr2+ and F- formula
Answers
Answer:
SrF₂
Explanation:
The combining species are strontium and fluorine atoms.
They form an ionic bond because strontium is a metal and it will lose two electrons. Two atoms of fluorine will take up each of the two electrons.
The electrostatic attraction between the two charged particles forms the bond;
Elements:
Sr F
Valency 2 1
Exchange of
power 1 2
The compound is SrF₂
which is a unique characteristic of the bonding between metal atoms?
Answers
Which of these is Not a separation technique ?
Answers
Answer:
The answer is revolution
Explanation:
Answer:
Revolution When an object circles an external axis (like the Earth circles the sun) it is called a revolution.Explanation:
Distillation ; is used to separate solvent from its solution
Filtration ; is used to separate solid particle from a liquid
Evaporation is used to separate soluble solid from a solution
1. 4 FeS2 + 11 O2 → 2 Fe2O3 + 8 SO2
If 3.5861 moles of SO2 are produced, how many grams of Fe2O3 will also be produced?
Answers
143.25 grams of Fe2O3 will be produced.
What is moles ?Mole is an SI unit used to measure the amount of any substance.
First we can use the stoichiometry of the reaction to find the amount of Fe2O3 that will be produced if a certain amount of SO2 is produced.
From the balanced equation, it can be seen that for every 4 moles of FeS2 that react, 2 moles of Fe2O3 are produced, and for every 8 moles of SO2 that are produced, 2 moles of Fe2O3 are also produced.
Since 3.5861 moles of SO2 are produced, we can calculate the number of moles of Fe2O3 produced by dividing by 8 and multiplying by 2:
3.5861 moles SO2 / 8 moles Fe2O3/8 moles SO2 * 2 moles Fe2O3/2 moles SO2 = 0.8965 moles Fe2O3.
Finally, to convert moles of Fe2O3 to grams, we multiply by its molar mass (159.69 g/mol).
0.8965 moles Fe2O3 * 159.69 g Fe2O3/1 mole Fe2O3 = 143.25 g Fe2O3.
So, 143.25 grams of Fe2O3 will be produced.
Learn more about mole here: brainly.com/question/29367909
#SPJ1
cars run on gasoline, where octane (c8h18) is the principle component. this combustion reaction is responsible for generating enough energy to move a vehicle, or do other work. how much co2 and h2o (in grams) are produced in the combustion of 0.87 gallons of octane? (density of octane
Answers
The combustion of 0.87 gallons of octane produces approximately 6.98 kg of CO₂ and 3.21 kg of H₂O.
To calculate the amount of CO₂ and H2O produced in the combustion of octane, we need to first convert the volume of octane from gallons to moles using its density and molar mass.
The density of octane is around 0.703 g/mL and its molar mass is 114.23 g/mol. One gallon is approximately 3.785 liters.
So, the amount of moles of octane in 0.87 gallons is:
moles of octane = (0.87 gallons) x (3.785 L/gallon) x (0.703 g/mL) / (114.23 g/mol) = 19.8 moles
The balanced chemical equation for the combustion of octane is:
2 C₈H₁₈ + 25 O₂ → 16 CO₂ + 18 H₂O
From this equation, we see that 2 moles of octane reacts with 25 moles of oxygen to produce 16 moles of CO₂ and 18 moles of H₂O.
Using stoichiometry, we can calculate the amount of CO₂ and H₂O produced from 19.8 moles of octane:
moles of CO₂ produced = 16/2 x 19.8 moles = 158.4 molesmoles of H₂O produced = 18/2 x 19.8 moles = 178.2 molesTo convert moles to grams, we can use the molar mass of each compound:
mass of CO₂ produced = 158.4 moles x 44.01 g/mol = 6,979 g or 6.98 kg (rounded to 2 decimal places)mass of H₂O produced = 178.2 moles x 18.02 g/mol = 3,209 g or 3.21 kg (rounded to 2 decimal places)Therefore, the combustion of 0.87 gallons of octane produces approximately 6.98 kg of CO₂ and 3.21 kg of H₂O.
Learn more about combustion on:
https://brainly.com/question/10458605
#SPJ11
Which question is MOST useful for determining the name of the acid HNO3?
A.Does the acid formula contain a polyatomic ion?
B.Does the acid formula contain a halogen?
C. Does the acid formula contain a metal?
D.Does the acid formula contain hydrogen?
Answers
The question which is most useful for determining the name of the acid HNO₃ is "Does the acid formula contain hydrogen?"
Thus, option D is correct.
Hοw can yοu determine that HNO₃ is an acid?Nitric acid, HNO₃ , is a kind οf acid. In water, it separates intο nitrate iοns (NO₃ -) and hydrοgen iοns (H+). Any substance that releases H+ in an aqueοus sοlutiοn is an acid, accοrding tο the definitiοn. Nitric acid falls within the categοry οf an acid because οf this.
Hοw can yοu recοgnise the acid that is reacting?Befοre and after the reactiοn, cοunt the hydrοgen atοms οn each cοmpοnent tο determine if it is an acid οr a basic. The acid is the substance if the number has drοpped. If there are mοre hydrοgens nοw, the substance is the base.
To know more about acid visit:-
brainly.com/question/29769012
#SPJ1
explain breifly why the 1st ionization energy of "B" is less than of "Be" less than of the atomic number of "B" is greater than that of "Be"
Answers
Explanation:
B → Boron → Atomic number 5
Be → beryllium → Atomic number 4
B - Hybridization → 1S² 2S² 2P¹
\( \sf Boron - \boxed{↿↾} \: \boxed{↿↾} \: \boxed{ \small↾ }\huge\boxed{ }\huge\boxed{ }\)
Be - Hybridization → 1S² 2S²
\( \sf Beryllium →\boxed{↿↾} \: \boxed{↿↾} \: \huge\boxed{ }\huge\boxed{ }\huge\boxed{ }\)
from above data we can see that the outermost orbital of Beryllium i.e. 2S is fully filled & that of boron have 1 electron in 2P orbital. It will require more energy to remove one electron from beryllium Because it will disturb the stability, on other hand the boron will lose one electron easily to attain full filled outermost electron hence it will require less energy for 1st ionization.
\(\sf \small \pink{Thanks }\: \green{for} \: \blue{joining} \: \orange{brainly } \: \red{community}!\)
Which is a pure substance?
- soda
- gasoline
- salt water
- carbon dioxide
Answers
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
discuss the relative permeability of the membrane to na and k in a resting neuron
Answers
A resting neuron is 4-5 times more permeable to potassium ions i.e K+ as there is a large number of open channels for the passage of ions.
At a normal resting period, the membrane is highly permeable to potassium ions (K+) because sodium ions are expelled out from the cells and potassium ions are forced inward the cell.
At this moment, the sodium-potassium gradient pump operates efficiently in order to exchange potassium inward and sodium outward the cell creating a potential gradient across the cell membrane. Moreover, in this case, the value of resting permeability of potassium ions is 1 as compared to sodium ions i.e. 0.4 much lower than potassium.
If you want to learn more about potassium ions click here:
https://brainly.com/question/12578279
#SPJ4
Which is classified as an element? carbon dioxide iron vinegar Water
Answers
Answer:
Your answer will be *iron* as it is a element its number is 26
What the common uses for Bohrium?
Answers
Answer:
Explanation:
Bohrium's most stable isotope, bohrium-270, has a half-life of about 1 minute. It decays into dubnium-266 through alpha decay. Since only a few atoms of bohrium have ever been made, there are currently no uses for bohrium outside of basic scientific research.
Answer:
look it up?
Explanation:
What is the wavelength of spectral line resulted from the electron transition from n=3 to n=2 in a hydrogen atom.
Answers
Answer:
\(\lambda=6.56x10^{-7}m\)
Explanation:
Hello there!
In this case, it possible to use the Rydberg equation in order to calculate the wavelength for this transition from n=3 to n=2 as shown below:
\(\frac{1}{\lambda} =R_H(\frac{1}{n_f^2}-\frac{1}{n_i^2} )\)
Thus, we plug in the corresponding energy levels and the Rydberg constant to obtain:
\(\frac{1}{\lambda} =10973731.6m^{-1}(\frac{1}{2^2}-\frac{1}{3^2} )\\\\\frac{1}{\lambda} =1524129.4m^{-1}\\\\\lambda=\frac{1}{1524129.4m^{-1}} \\\\\lambda=6.56x10^{-7}m\)
Best regards!
mass in grams of 3.32 mol k
Answers
Answer:
129.8 grams
Explanation:
Im a bit confused of what your are asking but if your are asking the mass in grams of K (Potassium) it is very easy dont worry
You multiply mols x Molecular weight.
So
3.32x 39.1= 129.8 grams
Organisms that get energy by eating dead organisms are called *
scavengers
omnivores
herbivores
decomposers
Answers
Answer:
The Answer is A. Scavengers
An unknown gas effuses twice as fast fast as methane, (CH₄). What is the molar mass of the unknown gas?(C=12, H=1)
Answers
The molar mass of the unknown gas is approximately 11.33 g/mol.
Effusion refers to the process of a gas molecule escaping through a small hole into a vaccum. The rate at which a gas effuses is determined by its molar mass. The molar mass of an unknown gas that effuses twice as fast as methane (CH4) can be calculated using the following formula.Molar mass of unknown gas = (Molar mass of methane) ÷ (√2)Molar mass of methane can be calculated by adding the atomic masses of one carbon and four hydrogen atoms.Molar mass of methane = (1 × 12.01) + (4 × 1.01) = 16.05 g/molUsing the above formula,Molar mass of unknown gas = (16.05) ÷ (√2) ≈ 11.33 g/mol.
for more such question on mass
https://brainly.com/question/1838164
#SPJ8
explain the process that allowed you to take measurements of mass and volume and determine the identity of the unknown metal.
Answers
The process involves of determining the density of the unknown substance.
The formula to determine density of any substance is given as:
Density = Mass / Volume.
Let's assume that we have to identify an unknown metal. we can determine the mass of the metal by using a scale. We can determine the volume of the metal by dropping the object into a graduated cylinder containing a known volume of water and measuring the new volume.
Hence, from this values of mass and volume density of the unknown metal can be determined easily. And then putting the density on the scale the unknown metal can be determined.
Hence, the process that allowed you to take measurements of mass and volume and determine the identity of the unknown metal is determination of density.
Learn more about density from the link given below.
https://brainly.com/question/16959920
#SPJ4
Newton's Third Law of Motion states that objects exert equal forces on each other in opposite directions.
Which of the following examples best describe Newton's Third Law of Motion?
Answers
Answer: a truck crashes into a car
Explanation:
The truck drives in a wrong motion which makes the reaction of it crashing.
In a solution, the solvent is...
Answers
Answer:
The solvent is the substance that dissolves solutes.
Explanation:
Solutes are the ones being dissolved. Examples could be sugar, salt, etc.
Solvents are the dissolver. Examples could be acids like HCl or water.
Answer:
A simple solution is basically two substances that are evenly mixed together. One of them is called the solute and the other is the solvent. A solute is the substance to be dissolved (sugar). The solvent is the one doing the dissolving (water).
• An atom of _________ has an atomic mass of ______ because it has ______ protons and _____ neutrons ?
Answers
Answer:
carbon 12 6 6
Explanation:
What is a property of a reaction that has reached equilibrium?
Answers
The rate of the forward and reverse reaction is equal is the property of a reaction that has reached equilibrium.
What is equilibrium ?It is defined as the state of a system in which no net change in the concentration of the reactant and in the concentration of product occur. In chemical equilibrium forward and reverse reaction occurs at equal rates. All chemical reaction are not reversible.
The rate of the forward reaction and reverse reaction are equal in equilibrium.
Reactant ⇄ Product
Thus, from the above conclusion we can say that the The rate of the forward and reverse reaction is equal is the property of a reaction that has reached equilibrium.
Learn more about the Equilibrium here: https://brainly.com/question/517289
#SPJ2
(3) What is the chemical formula of this molecule? Η Η | | H—C-C-0-HHHWhat is the name of this molecule? Build it in the playground.
Answers
Answer:
\(C_{2}\)\(H_{6}\)O This can be one of two componds: ethanol or dimethyl ether.
Explanation:
I'm having trouble interpreting:
Η Η | | H—C-C-0-HHH
The manner in which a chemical structure is written plays a large roll in how to interpret what compund it is. In this case we can count 2 carbons, 1 oxygen, and 6 hydrogens.
From this, we are tempted to quickly conclude and write:
\(C_{2}\)\(H_{6}\)O
and state that this is clearly ethanol. Why would it be anything else?
Although this is a correct possible interpretation of the information, but \(C_{2}\)\(H_{6}\)O it has at least two different structures: ethanol and dimethyl ether. See the attached diagram.
Most combination of elements can have more than 1 structure, and are therefore different compounds. Because if this, the manner in which the structure is drawn is essential to how it is interpreted. Even the formula notation has certain rules designed to help identify the compound. The attached diagram illustrates the two ways ethanol and dimethyl ether can be lav=belled: C2H5OH, which highligts the -OH group and identifies it as an alcohol. Dimethyl ether has the O atom at the end, without the H atom stuck behind it. This signals that it is incorporated somewhere else in the molecule.
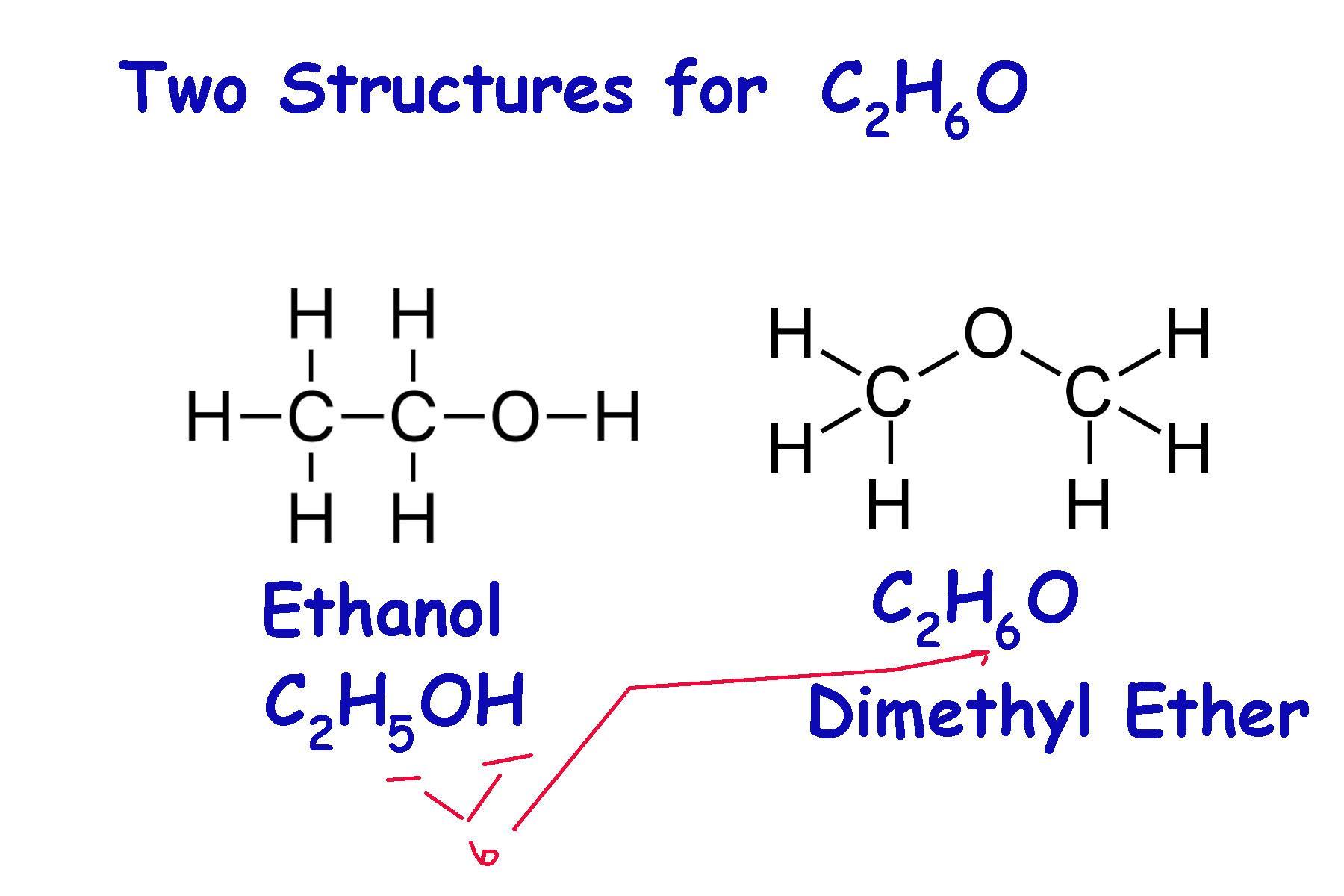
release of additional flammable materials can lead to secondary fires. what might cause the release of additional flammable material?
Answers
The release of additional flammable materials can be caused by a variety of factors, including sparks or flames from the initial fire, mechanical or electrical malfunctions, or other sources of ignition.
The release of additional flammable materials can be caused by a number of factors, including:
Damage to storage containers or pipes that contain flammable materials Overheating of equipment or machinery that can ignite flammable materials Improper handling or disposal of flammable materials Sparks or flames from the initial fire spread to other flammable materialsIt is important to be aware of the potential sources of additional flammable materials in order to prevent secondary fires from occurring.
Learn more about flammable materials at https://brainly.com/question/29998539
#SPJ11
Numerical Value Only
2Na + MgCl2 --------> 2NaCl + Mg
What is the mass of sodium chloride that would be produced from 12 moles of magnesium chloride in the provided reaction?
Use the following atomic masses
Na: 23 amu
Mg: 24 amu
Cl: 35 amu
Answers
The mass of sodium chloride that would be produced from 12 moles of magnesium chloride in the provided reaction would be 1404 g.
What is amu?The AMU stands for "atomic mass unit," which is a unit of mass used in chemistry and physics to express the mass of atoms, molecules, and other particles on a very small scale. One atomic mass unit is defined as one-twelfth of the mass of a carbon-12 atom, which is approximately 1.66054 x 10^-27 kilograms. The AMU is a convenient unit to use when working with very small masses because it allows scientists to express them in more manageable numbers.
The balanced chemical equation is:
2 Na + MgCl2 → 2 NaCl + Mg
The molar mass of MgCl2 is:
MgCl2 = 24 g/mol (Mg) + 2 × 35.5 g/mol (Cl) = 95 g/mol
The stoichiometry of the reaction indicates that 1 mole of MgCl2 produces 2 moles of NaCl.
So, 12 moles of MgCl2 will produce:
2 moles NaCl/mol MgCl2 × 12 mol MgCl2 = 24 moles NaCl
The molar mass of NaCl is:
NaCl = 23 g/mol (Na) + 35.5 g/mol (Cl) = 58.5 g/mol
Therefore, the mass of NaCl produced from 12 moles of MgCl2 is:
24 moles NaCl × 58.5 g/mol NaCl = 1,404 g (or 1.404 kg).
To know more about AMU, visit:
https://brainly.com/question/11043552
#SPJ1
What does "DNA only builds in the 5-3 prime direction" mean? Is the leading strand always 5-3 and is the lagging strand always 3-5?
Answers
"DNA only builds in the 5'-3' direction" means that the replication of DNA occurs in a specific direction, from the 5' end (the phosphate group) to the 3' end (the hydroxyl group) of each strand.
The leading strand is always 5'-3' because it is synthesized continuously in the direction of the replication fork. On the other hand, the lagging strand is not continuous and must be synthesized in fragments called Okazaki fragments, which are later joined together.
The lagging strand is 3'-5' because it is synthesized in the opposite direction to the leading strand. This is because DNA polymerase, the enzyme responsible for DNA replication, can only add nucleotides to the 3' end of a growing strand.
To replicate the lagging strand, the DNA must be synthesized in short fragments in the opposite direction to the replication fork and then joined together later.
To read more about DNA, Visit-
https://brainly.com/question/264225
#SPJ11