5. On a 92 point test, a student scored 87 points. What is their percent error?
Answers
Answer:
Explanation:
5.7471264367816%
Related Questions
what is the concentration of OH− in a solution with a pOH value of 6.09
Answers
Answer:
I'm not sure
Explanation:
I'm just not sure
Which pair of aqueous solutions, when mixed, will form a precipitate? a. NH4Cl and NaBr
b. NaNO3 and AgC2H3O2 c. CaCl2 and CsI
d. NaCl and Pb(NO3)2
Answers
Only the pair of solutions in option d will form a precipitate upon mixing.The pair of aqueous solutions that will form a precipitate upon mixing is d. NaCl and Pb(NO3)2.
This is because the combination of these two solutions will result in the formation of insoluble lead chloride (PbCl2) precipitate, which is white in color. The reaction that takes place is:
2NaCl (aq) + Pb(NO3)2 (aq) → 2NaNO3 (aq) + PbCl2 (s)
The other options, a. NH4Cl and NaBr, b. NaNO3 and AgC2H3O2, and c. CaCl2 and CsI do not result in the formation of a precipitate when mixed. When NH4Cl and NaBr are mixed, they will form a clear and colorless solution as both are highly soluble in water. Similarly, NaNO3 and AgC2H3O2 will also form a clear and colorless solution, as both are highly soluble in water. Finally, CaCl2 and CsI will form a clear and colorless solution as both salts are highly soluble in water. Therefore, only the pair of solutions in option d will form a precipitate upon mixing.
learn more about solutions
https://brainly.com/question/1024087
#SPJ11
the general chemical composition of various window glasses has been found to be relatively uniform among various manufacturers and therefore offers no basis for identifying individual samples.truefalse
Answers
The statement "the general chemical composition of various window glasses has been found to be relatively uniform among various manufacturers and therefore offers no basis for identifying individual samples" is True.
Glass is a commonly used material that is used in a wide range of applications, including window panes. The chemical composition of window glass is relatively constant among different manufacturers, according to research. Therefore, it does not provide a reliable method for identifying various glass samples based on their chemical composition.
The given statement "the general chemical composition of various window glasses has been found to be relatively uniform among various manufacturers and therefore offers no basis for identifying individual samples" is true, because the chemical composition of glass is constant, researchers employ alternative techniques to distinguish between various glass samples. Some of these techniques include the use of trace elements, the age of the glass, and other physical characteristics such as refractive index and density.
Therefore the given statement is true.
Learn more about glass on:
https://brainly.com/question/465042
#SPJ11
what is the importance of filtering kmno4 solution and why do we use glass-wool instead of filter-paper?
Answers
so.. the answer is “ to retain any presence of MnO[sub]2[/sub]that may drop out of solution during filtration.”
hope this helps!
The galaxy we live in is the Milky Way galaxy, and it is a spiral galaxy. Galaxies contains billions of which of the following?
A.Smaller galaxies
B.Black holes
C.Planets
D.Stars
Need help ASAP
Answers
Answer:
D. Stars is the correct answer
Please give my answer the brainliest...
Jose adds five grams of a chemical to one liter of liquid and observes a reaction. If the reaction is endothermic, what will happen?
A
The liquid's mass increases; therefore, the volume of the liquid will increase
B
The liquids' mass decreases; therefore, the volume of the liquid will decrease.
С
Energy is released during the reaction; therefore, the temperature of the liquid will increase.
Energy is absorbed during the reaction; therefore, the temperature of the liquid will decrease.
D
Answers
Answer:
D. Energy is absorbed during the reaction; therefore, the temperature of the liquid will decrease
Explanation:
In a chemical reaction, the reaction is said to be ENDOTHERMIC if the reaction utilizes or uses energy instead of given it off. In this reaction, Jose adds five grams of a chemical to one liter of liquid and observes the reaction.
According to this question, the reaction is ENDOTHERMIC. This means that energy is absorbed or used up during the reaction; therefore, the temperature of the liquid will decrease i.e. the liquid used in the reaction will become COLDER.
what is the major organic product obtained from the following reaction naoh h2o heat o double bond phenyl
Answers
The reaction you are referring to involves the use of NaOH (sodium hydroxide), H2O (water), and heat with a phenol compound that has a double bond to an oxygen (O) atom, which is an aromatic ketone. In this reaction, the major organic product obtained is a phenol.
The presence of a strong base, NaOH, and heat causes the ketone to undergo a base-catalyzed alpha-hydroxylation reaction. The hydroxide ion (OH-) from NaOH attacks the alpha-carbon, forming a carbanion intermediate. The carbanion then reacts with water, which acts as a nucleophile, to form an alpha-hydroxyketone.
Subsequently, the alpha-hydroxyketone undergoes a tautomeric shift, a type of isomerization reaction. The tautomeric shift involves the movement of a proton and the migration of a double bond to form a more stable compound. In this case, the keto form (aromatic ketone) converts into its enol form (phenol).
The phenol is the major organic product in this reaction due to the increased stability provided by the aromatic ring and the hydrogen bonding capabilities of the hydroxyl group.
For more such questions on phenol, click on:
https://brainly.com/question/9512716
#SPJ11
At 9°C a gas has a volume of 6.17 L. What is its volume when the gas is at standard temperature?
Answers
Answer:
V₂ = 5.97 L
Explanation:
Given data:
Initial temperature = 9°C (9+273 = 282 K)
Initial volume of gas = 6.17 L
Final volume of gas = ?
Final temperature = standard = 273 K
Solution:
Formula:
The Charles Law will be apply to solve the given problem.
According to this law, 'the volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure'
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
V₂ = V₁T₂/T₁
V₂ = 6.17 L × 273K / 282 k
V₂ = 1684.41 L.K / 282 K
V₂ = 5.97 L
An XRD is ‘set up’ with a Mo target and filters to fully attenuate Kb radiation. Refer to Table 1. Determine the monochromatic x-ray wavelength:
l = 0.71144
This XRD scan of a pure but unknown element having BCC or FCC cubic crystal structure reveals 1st and 2nd peaks at 2qA = 11.622558513411379 deg. and 2qB = 13.428288268863643 deg., respectively.
What is the cubic crystal structure? _____FCC
What is the lattice constant in Angstroms? ___
Refer to AA_Crystal Structures of Some Elements.pdf posted to zip folder and determine the unknown.
Unknown Element______________
Answers
Based on the data provided, (a) the cubic crystal structure is FCC, (b) the lattice constant in Angstroms is 0.3615 and (c) the unknown element is Nickel.
Here is the solution:
The first peak at 2qA = 11.622558513411379 degrees corresponds to the (111) reflection, which is the most intense reflection for FCC crystals.
The second peak at 2qB = 13.428288268863643 degrees corresponds to the (200) reflection.
The Bragg equation is:
2d sin(θ) = λ
where d is the lattice spacing, θ is the scattering angle, and λ is the wavelength of the X-ray.
Solving for d, we get:
d = λ / 2 sin(θ)
Substituting the values for λ and θ, we get:
d = 0.71144 Å / 2 sin(11.622558513411379°)
≈ 0.3615 Å
The lattice constant for FCC Nickel is 0.3524 Å, which is very close to the calculated value of 0.3615 Å. Therefore, the unknown element is Nickel.
Thus, (a) the cubic crystal structure is FCC, (b) the lattice constant in Angstroms is 0.3615 and (c) the unknown element is Nickel.
To learn more about FCC crystal :
https://brainly.com/question/12977980
#SPJ11
What is reduced and what is oxidized in the reaction between a monosaccharide and a ferric ion?
Answers
In the reaction between a monosaccharide and a ferric ion, The carbonyl carbon is oxidized to a carboxyl group, the cupric ion is reduced.
Chemical reactions often involve color changes, temperature changes, gas evolution, or precipitate formation. Simple examples of everyday reactions are digestion, combustion, and cooking. The definition of reaction is a reaction. An example of a reaction is someone stopping their car at a stop sign. noun. response to stimuli.
The five basic types of chemical reactions are combination, decomposition, single exchange, double exchange, and combustion. By analyzing the reactants and products of a particular reaction, we can classify them into one of these categories. Some responses fit into multiple categories. It is modeled after the old Italian reaction, the French response, derived from the medieval Latin response (nominative to react). It is a noun of action formed in Late Latin from the past participle stem of the Latin reader.
Learn more about the reaction here
https://brainly.com/question/11231920
#SPJ4
What is oxidized and what is reduced in this reaction? Cu(s) 2AgNO3(aq) → Cu(NO3)2(aq) 2Ag(s).
Answers
In the given reaction:
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
Copper (Cu) is being oxidized, and silver (Ag) ions are being reduced.
To determine oxidation and reduction, we look at the changes in oxidation states (or oxidation numbers) of the elements involved in the reaction.
In this reaction, the oxidation state of copper (Cu) changes from 0 in its elemental form (Cu(s)) to +2 in the copper(II) nitrate compound (Cu(NO3)2(aq)). This indicates that copper has lost electrons and has been oxidized.
On the other hand, the silver ions (Ag⁺) in the silver nitrate solution (AgNO3(aq)) are being reduced. The silver ions have an oxidation state of +1 in the compound, but when they react with copper, they are reduced to elemental silver (Ag(s)) with an oxidation state of 0. This reduction process involves the gain of electrons.
Therefore, in the given reaction, copper is oxidized and silver ions are reduced.
To know more about oxidation, visit
https://brainly.com/question/15578795
#SPJ11
Suppose 5.00g of Zn metal is completely consumed in an HCl solution to produce zinc(II) choride (ZnCl2) and hydrogen gas (H2) according to the following reaction: Zn(s) + 2HCl(aq) -----> ZnCl2(aq) + H2(g)
Answers
If 5.00g of Zn metal is completely consumed in an HCl solution to produce Zinc (III) chloride (ZnCl₂) and Hydrogen gas (H₂), 0.0764 moles of ZnCl₂ will be produced.
To find the number of moles of ZnCl₂ produced, it is required to calculating the number of moles of Zn consumed.
According to question:
Mass of zinc = 5.00 g
Molar mass of zinc = 65.38 g/mol
By using the molar mass of Zn, find the number of moles of Zn:
Number of moles of zinc = Mass of Zn ÷ Molar mass of Zn
= 5.00 g ÷ 65.38 g/mol
= 0.0764
The stoichiometric ratio of Zn and ZnCl₂ is 1:1, according to the balanced equation. As a result, the number of moles of ZnCl₂ produced will be 0.0763 mol.
Thus, 0.0764 moles of ZnCl₂ will be produced.
Learn more about moles, here:
https://brainly.com/question/31646807
#SPJ1
The given question is incomplete, so the most probable complete question is,
Suppose 5.00g of Zn metal is completely consumed in an HCl solution to produce Zinc (III) chloride (ZnCl₂) and Hydrogen gas (H₂) according to the following reaction: Zn(s) + 2HCl(aq) --> ZnCl₂(aq) + H₂(g). How many moles of ZnCl₂?
what is hard water and soft water
Answers
Answer:
Hard water is water that has high mineral content, Soft water is free from dissolved salts of such metals as calcium, iron, or magnesium
Explanation:
What is a constraint on the solar oven design?
A. The solar oven must heat up quickly
B. The design should look like the provided diagram
C. The solar work in any kind of weather
D. The materials may cost no more than sixty dollars
Answers
Answer:
D
Explanation:
I may need a photo but D seemed the most logical
Identify a substance which can reduce Br2(l) to Br−(aq) but cannot reduce I2(s) to I−(aq).
Answers
Ag (Silver) can reduce Br₂ (I) to Br- (aq) but it cannot reduce I₂ (s) to I-(aq) because silver is a stronger oxidizing agent and I₂ can be reduced with the help of hydrogen Peroxide (H₂O₂).
In case of bromine-
The chlorine gains these electrons, so it is the oxidizing agent (becoming reduced to Cl– ions). The bromine has given electrons to the chlorine, so the bromide ions are the reducing agent (becoming oxidized to Br atoms, which form Br₂).
In case of Iodine-
HI to I2 is oxidation. The I– ion in HI loses an electron to become an I atom, which then joins with another I atom to form an I2 molecule. here oxidation takes place therefore Ag which is a oxidizing agent, can't help.
Learn more about Oxidizing Agent here-
https://brainly.com/question/10547418
#SPJ4
Compare and contrast the elements Potassium, K, and Neon, Ne. Use at least ten comparisons or descriptions.
I need this done asap!!!
Answers
Answer:
K is a group 1 element while Ne is a group 0 element
K is a good reducing agent while Ne is inert
K is metal while Ne is a gas
K has one valence electron while Ne has no valence electron
Explanation:
I need help with this question please someone tell me the answer for this

Answers
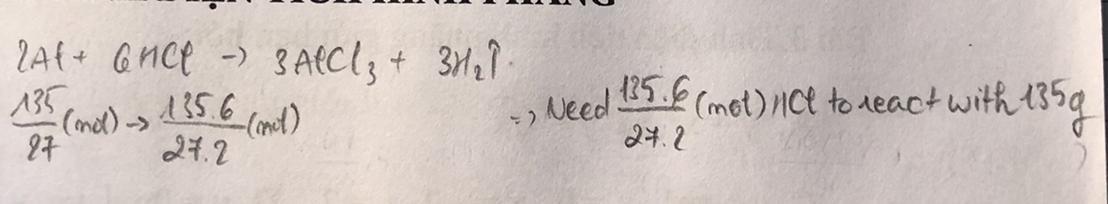
Indicate the charge the following elements as they achieve the noble gas configuration.
Ga O Br P Rb As
S Mg Al Se Li I
Answers
Answer:
See explanation
Explanation:
Ga is in group 13 hence it must loose three electrons to form Ga^3+ in order to achieve the noble gas configuration because it has three electrons on its outermost shell.
O is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form O^2- since oxygen has six electrons on its outermost shell.
Br in group 17 has seven electrons in its outermost shell hence it must form Br^- (gain one electron) in order to attain the noble gas configuration.
P in group 15 must accept three electrons and form P^3- in order to attain the noble gas configuration since it has five electrons on its outermost shell.
S is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form S^2- since sulphur has six electrons on its outermost shell.
Mg in group 2 has two electrons on its outermost shell and must loose both to attain the noble gas configuration forming Mg^2+.
Al is in group 13 hence it must loose three electrons to form Al^3+ in order to achieve the noble gas configuration because it has three electrons on its outermost shell.
Se is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form Se^2- since selenium has six electrons on its outermost shell.
Lithium is in group 1 and must loose its only outermost electron in order to attain the noble gas configuration to form Li^+.
Rb is in group 1 and must loose its only outermost electron in order to attain the noble gas configuration to form Rb^+.
As in group 15 must accept three electrons and form As^3- in order to attain the noble gas configuration since it has five electrons on its outermost shell.
I in group 17 has seven electrons in its outermost shell hence it must form I^- (gain one electron) in order to attain the noble gas configuration.
look at 3 or 4 other atoms using the simulation. Do any of them ahve a whole number for atomic mass?
Answers
Answer:
There are three isotopes of silicons. They have masses numbers of 28, 29 and 30. The average amount of silicons is 28.086alum
Explanation:
I learned this in class three days ago so I hope this is helpful i'm in high school ask me for help when ever you want Iĺl help you
Write a complete, balanced chemical equation where tin metal reacts with aqueous hydrochloric acid to produce tin(II) chloride and hydrogen gas. Include states.
From the equation, which element is oxidized, and which element is reduced?
Answers
Answer:
Zn(s)+2HCl(aq)→ZnCl2(aq)+H2(g)
Explanation:
The net reaction is Z n ( s ) + 2 H + ( a q ) → Z n 2 + ( a q ) + H 2 ( g ) The C l − ions are spectators - they don't change.
Carbon tetrachlordie (CCI4) is ___
A. iconic
B. Covalent

Answers
Why? Because Carbon is a non-metal, and Chlorine is a non-metal. Two non-metal elements make a covalent or molecular compound.
Does an electron cause an electromagnetic wave to vibrate
Answers
Yes, an electron cause an electromagnetic wave to vibrate.
What is an electromagnetic wave?One of the waves is propagated by simultaneous periodic variations of electric and magnetic field intensity and that includes radio waves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
Electromagnetic waves are produced when something vibrates—an electric charge that moves back and forth.
When an electric charge vibrates, the electric field around it changes. Because the electric charge is in motion, it also has a magnetic field around it.
This magnetic field also changes as the charge vibrates.
Therefore, an electron causes an electromagnetic wave to vibrate.
Learn more about the electromagnetic wave here:
https://brainly.com/question/3596193
#SPJ1
Standard fire hydrants should not be installed on water mains smaller than?
a) 4
b) 6
c) 8
d) 10
Answers
Standard fire hydrants should not be installed on water mains smaller than 6 inches in size.
Fire Hydrants and Branches:
1. Gridironing of Public Water Mains: Whenever possible, gridironing of public water mains shall be planned so that not more than one fire hydrant will be installed on a six-inch (6″) diameter water main between intersecting mains, and not more than two (2) fire hydrants installed on an eight-inch (8″) diameter water main between intersecting mains.
2. High Value Areas: In industrial, warehouse, institutional, shopping center, or other high value areas within or outside the principal business district, there shall be one or two (2) fire hydrants at each street intersection, depending upon the character of the area, with intermediate fire hydrants placed so that they are not over three hundred feet (300′) apart. In general, depending upon the area’s characteristics, the average area to be served by each fire hydrant shall be from eighty thousand (80,000) to ninety thousand (90,000) square feet.
3. Residential Areas: In residential areas there shall be one fire hydrant installed at each street intersection with intermediate fire hydrants located so that said fire hydrants are spaced not over three hundred feet (300′) apart. In general, depending upon the area’s characteristics, the average area to be served by each fire hydrant shall not exceed one hundred ten thousand (110,000) square feet.
4. Fire Hydrant Branches: Fire hydrant branches shall have a minimum diameter of six inches (6″). In all cases a valve shall be installed on each fire hydrant branch and in no case shall the valve be of smaller diameter than the fire hydrant branch. Branch valves shall be situated not less than eighteen inches (18″) or more than twenty-four inches (24″) from the branch feeder main.
To know more about Fire please click:-
https://brainly.com/question/28901632
#SPJ11
If the velocity of a bicycle is 10m/s,how long will it take to cover a distance of 18km?
Answers
Answer:
t = 1800 s
Explanation:
Given data:
Velocity of bicycle = 10 m/s
Distance cover = 18 Km
Time taken = ?
Solution:
Distance cover = 18 Km (18km×1000m/1km=18000 m)
Formula:
Velocity = distance/ time
by putting values,
10 m/s = 18000 m/ t
t = 18000 m/ 10 m/s
t = 1800 s
Why does the battery give a reading of 9V even though there are no electrons flowing around the circuit?
Answers
Answer:
because battery have it's own voltage in it's composition
Why can't sound waves travel through space?
Answers
how many moles are in 32.3g of calcium phosphate
Answers
Answer: the formula mass of calcium phosphate [Ca3(PO4)2] is 310.177 amu, so its molar mass is 310.177 g/mol. This is the mass of calcium phosphate that contains 6.022 × 1023 formula units.
Answer: 310.177
what is the chemical portion of neuron signal transmission?
Answers
The chemical portion of neuron signal transmission is called neurotransmission.
This process involves the release of chemical messengers called neurotransmitters from the presynaptic neuron (the neuron sending the signal) into the synaptic cleft (the space between the presynaptic and postsynaptic neurons). The neurotransmitters then bind to receptors on the postsynaptic neuron (the neuron receiving the signal), causing a change in the electrical activity of the postsynaptic neuron.
This change in electrical activity can either excite or inhibit the postsynaptic neuron, leading to either an increase or decrease in the likelihood that the postsynaptic neuron will fire an action potential. Neurotransmission is a crucial component of neuronal communication and is involved in a wide range of physiological processes, including sensation, movement, cognition, and emotion.
To know more about Neurotransmission click here:
https://brainly.com/question/28562598#
#SPJ11
A pan containing 20.0 grams of water was allowed to cool from a temperature of 95.0 °C. If the amount of heat released is 1,200 joules, what is the approximate final temperature of the water? (5 points)
75 °C
78 °C
81 °C
87 °C
Answers
Answer:
78° C.
Explanation:
The final temperature of water is 81℃ , if it is allowed to cool from a temperature of 95 and it releases heat of 1200J.
What is the final temperature of water?In the following question we will apply specific heat Capacity equation
q=mct Δt
q= 1200J m= 20 g c= 4.186 T₁= 95 ℃
1200= 20 ✕4.186(95-T₂)
T₂=81℃
Hence, The water cools to 81℃ temperature
Learn about specific heat capacity
https://brainly.com/question/1747943
#SPJ2
What is the name of the ability to use up energy in one second
Answers
Answer:
Work
Explanation:
Work is defined as the ability to use energy in one second and its SI unit is same as energy that is joule.
Work refers to the energy utilized to displace an object over a distance by an external force in one direction and in given time period which can be one second as well.
Hence, the correct answer is "work".